Stem cell-like Xenopus Embryonic Explants to Study Early Neural Developmental Features In Vitro and In Vivo
Summary
In Xenopus embryos, cells from the roof of the blastocoel are pluripotent and can be programmed to generate various tissues. Here, we describe protocols to use amphibian blastocoel roof explants as an assay system to investigate key in vivo and in vitro features of early neural development.
Abstract
Understanding the genetic programs underlying neural development is an important goal of developmental and stem cell biology. In the amphibian blastula, cells from the roof of the blastocoel are pluripotent. These cells can be isolated, and programmed to generate various tissues through manipulation of genes expression or induction by morphogens. In this manuscript protocols are described for the use of Xenopus laevis blastocoel roof explants as an assay system to investigate key in vivo and in vitro features of early neural development. These protocols allow the investigation of fate acquisition, cell migration behaviors, and cell autonomous and non-autonomous properties. The blastocoel roof explants can be cultured in a serum-free defined medium and grafted into host embryos. This transplantation into an embryo allows the investigation of the long-term lineage commitment, the inductive properties, and the behavior of transplanted cells in vivo. These assays can be exploited to investigate molecular mechanisms, cellular processes and gene regulatory networks underlying neural development. In the context of regenerative medicine, these assays provide a means to generate neural-derived cell types in vitro that could be used in drug screening.
Introduction
The vertebrate nervous system emerges from the neural plate as a homogeneous layer of neuroepithelial cells. Understanding how developmental programs are induced, encoded, and established during regionalization of the neural plate is, at present, a major goal in developmental biology. Compared to other systems, the experimentally amenable Xenopus embryo is a model of choice for analyzing early steps of neural development1,2. It is easy to obtain large numbers of embryos, and external development gives access to the very first steps of neurulation3. Many tools are available to experimentally manipulate Xenopuslaevis (X. laevis) embryonic development. Micro-injection of mRNAs or morpholinos (MO), including inducible MOs, together with biochemical and pharmacological tools, allows controlled gain of function (GOF) and loss of function (LOF) and specific alteration of signaling pathways4,5. The blastocoel roof ectoderm, located around the animal pole of a blastula, or a very early gastrula embryo, and referred to as the 'Animal Cap' (AC), is a source of pluripotent cells that can be programmed by manipulation of gene expression prior to explants preparation. In this manuscript are detailed protocols to use X. laevis AC explants to test in vitro and in vivo molecular mechanisms and cellular processes underlying neural development.
A technique is presented, allowing fine observation of gene expression patterns in a Xenopus tadpole neural tube, a preliminary step in the identification of fate determination cues. Whereas the observation of flat-mounted tissues is commonly used in the study of chick embryos6, it has not been properly described in Xenopus. Manipulation of gene expression by injecting synthetic mRNA or MO into the blastomeres of 2 or 4 cell stage embryos allows programming of AC explants4. For example inhibition of the Bone Morphogenetic Protein (BMP) pathway by expression of the anti-BMP factor Noggin, gives a neural identity to AC cells3. The protocol is detailed for performing local and time-controlled exposure of AC explants to extrinsic cues via direct contact with an anion exchange resin bead. Finally a technique is described for testing developmental features of neural progenitors in vivo by transplantation of mixed explants prepared from distinct programmed cells dissociated and re-associated.
The frog embryo is a powerful model to study early vertebrate neural development. Combining manipulation of gene expression to explant in vitro cultures provides important information in the study of neuroepithelium regionalization, proliferation, and morphogenesis7-12. The programming of AC explants permitted development of a functional heart ex vivo13,14. The use of explant grafting15 led to the identification of the minimal transcriptional switch inducing the neural crest differentiation program16. The zona limitans intrathalamica (ZLI) is a signaling center that secretes sonic hedgehog (Shh) to control the growth and regionalization of the caudal forebrain. When continuously exposed to Shh, neuroepithelial cells coexpressing the three transcription factor genes – barH-like homeobox-2(barhl2),orthodenticle-2 (otx2) and iroquois-3 (irx3) – acquire two characteristics of the ZLI compartment: the competence to express shh, and the ability to segregate from anterior neural plate cells. As a model system, the induction of a ZLI fate into neuroepithelial cells will be presented8.
These protocols aim at providing simple, cheap, and efficient tools for developmental biologists and other researchers to explore the fundamental mechanisms of key neural cell behaviors. These protocols are very versatile and allow the investigation of a large range of extrinsic and intrinsic neural determination cues. It permits long term in vivo analysis of neural lineage commitment, inductive interactions and cell behaviors.
Protocol
Experiments comply with National and European regulation on the protection of animals used for scientific purposes and with internationally established principles of replacement, reduction and refinement.
1. Flat-mounting of Xenopus laevis Tadpoles Anterior Neural Tube After Whole-mount In Situ Hybridization
- Obtain X. laevis embryos according to standard procedures4 and age them until they reach neurula stage 26 and older (according to Nieuwkoop and Faber developmental table17.
- Fix X. laevis tadpoles by incubating them in a solution of 4% paraformaldehyde (PFA). Rock and rotate the embryos in a 2 ml glass vial , 1 to 1.5 hr at RT for stage 26 to stage 38 embryos, 2 hr at RT for embryos at later stages.
CAUTION: PFA is toxic by contact and a suspected carcinogen. It should be manipulated under a hood.
NOTE: One X. laevis brood is usually fixed in a 2 ml glass vial but a larger glass vial can be used. - Wash the embryos in the same vial using 1x PBS with 0.1% Tween (PBT), 3 min at RT, twice.
NOTE: Unless otherwise specify the PBS used is with or without calcium and magnesium. - Dehydrate the embryos in 100% methanol (MeOH) for at least 12 hr at RT in the same vial. Change the MeOH 100% at least twice under the hood using a plastic micropipette.
NOTE: The MeOH turns slightly yellow as lipids dissolved in it. Caution the MeOH is toxic by inhalation and should be manipulated under a hood. - Rehydrate the embryos using the same vial through a graded series of MeOH baths: MeOH 75% in PBS, MeOH 50% in PBS, MeOH 25% in PBS, PBS twice, each bath 5-10 min at RT. The MeOH solutions are changed under the hood using a plastic micropipette.
- Using a plastic pipette, transfer the embryo to be dissected in a 60 mm Petri dish filled to the top with PBT. Using two fine forceps carefully remove the eyes by inserting one fine forceps between the eyes and the neural tube, at the level of the optic stalk. Detach the eyes from the neural tube. Carefully introduce the forceps below the ectoderm overlying the neural tube. With care, peel off the ectoderm starting from behind the head and discard it.
- Perform a double or single ISH using digoxigenin-labeled or fluorescein-labeled probes as previously described18,19.
- After the ISH transfer the embryos in a new 60 mm Petri dish filled to the top with PBT using a plastic pipette.
- Using fine forceps carefully detach the neural tube from the rest of the embryo. At this stage, the anterior neural tube separates from the posterior neural tube. Carefully detach remaining parts of the ectoderm overlying the neural tube, the notochord that is loosely attached below the neural tube, the otic vesicles and remaining parts of the mesoderm. Ensure that the neural tube is devoid of any appendices at this stage.
NOTE: To avoid damage to the neural tube perform all neural tube/embryo transfer with a plastic transfer pipette or a micropipette if necessary with a tip cut at its end. The diameter of the tip end is adjusted to the size of the neural tube. - Transfer the dissected neural tube (see note step 1.9) in a 1.5 ml tube filled with 50% glycerol diluted in PBS. Wait until the neural tubes have fallen to the bottom of the glycerol solution, usually O/N at 4 °C.
- Remove the solution of 50% glycerol/PBS using a micropipette P1000 and add in the same 1.5 ml tube a solution of 90% glycerol/PBS using a micropipette P1000. Wait until the neural tubes fall to the bottom of the 90% glycerol/PBS solution, usually O/N at 4 °C.
NOTE: for long term storage, use 90% glycerol/PBS plus antibiotics to prevent bacterial development. - Transfer the neural tubes on a glass plate, or a microscope slide with a plastic transfer pipette.
- Dissect the neural tubes along the dorsal and ventral midlines using tungsten needles. During that step the neural tubes are kept in 90% glycerol/PBS.
- Mount the two sides of the neural tube in 90% glycerol/PBS using reinforcement rings covered with a glass coverslip.
- Fix the coverslips with varnish and keep at 4 °C.
2. Animal Cap Explants Induction Using Anion Exchange Resin Beads
- Transfer 100 µl of anion exchange resin beads into a 2 ml tube using a micropipette P1000. Wash the beads at least five consecutive times with sterile distilled water. Allow the beads to sediment at the bottom of the tube and replace the sterile distilled water using a micropipette P1000. Do not touch the beads.
- Let the beads soak O/N in a 2 ml tube filled with sterile distilled water with Bovine Serum Albumin (BSA) (10 mg/ ml) at 4 °C.
- Two hr before collecting the ACs, place half of the beads into a new 1.5 ml tube using a micropipette P1000. Replace the sterile distilled water with 500 µl of the medium containing the molecule to be tested for its inductive potential, or known to induce a specific fate. Keep the other half of the beads, as they will be used as a negative control.
- Incubate the beads at 4 °C for at least 2 hr.
- Carry out all subsequent steps under the stereomicroscope and perform all AC transfer with a micropipette.
- Transfer blastula or very early gastrula embryos into PBS with calcium and magnesium complemented with 0.2% BSA using a plastic transfer pipette.
NOTE: Embryos developing at 12 °C reach blastula and gastrula stages in 20 to 24 hr. - Remove the embryo's vitelline membrane using forceps as previously described20. Fix the embryo with one forceps. Pinch the vitelline membrane with the side of the other forceps. Holding the membrane, slowly peel it off the embryo. Perform this procedure on the ventral (vegetal) side of the embryo. Do not damage the animal side of the embryo.
- Isolate small ACs as previously described21. The animal pole is the embryo's pigmented part. Using fine forceps cut out a small square of tissue out of the animal pole. The AC tissue contains only the ectodermal cells. Ensure that the tissue is of uniform thickness. If not, remove the AC from the analysis.
- Place each AC in a Terasaki multiwell plates in 0.5x Modified Barth's Saline (MBS)4. Place the AC into the well with its pigmented animal side down, in contact with the round bottom of the well.
NOTE: coating pipettes with a 0.1% BSA solution prevents the adhesion of small pieces of adhesive tissue to the plastic. - Place one bead on each cap using a micropipette P20. If needed, use forceps to carefully place the bead in the center of the cap.
NOTE: When soaked in a conditioned medium, the beads are colored in pink. Select the most colored beads. - Incubate at RT (18 to 22 °C) for 6 hr without moving the Terasaki multiwell plate. The bead sticks to the AC in less than 2 hr.
- Place the Terasaki multiwell plate on top of papers soaked in water. Cover the plate with a plastic container.
NOTE: This 'humidity chamber' prevents premature evaporation of the wells. - Culture the ACs in 0.5x MBS or 3/4 NAM (see step 4.1) in the humidity chamber at 15-20 °C until the sibling embryos have reached the correct stage to test the effect of your factor.
- Fix the ACs for 1 hr in freshly prepared 4% PFA. Dehydrate the ACs in 100% MeOH as previously described (Step 1.4).
3. Animal Cap Cell Dissociation and Reaggregation Before Grafting in a Xenopuslaevis Neurula
- Coat petri dishes (60 mm) or 12 wells plates with 3% agarose in sterile water or in PBS without calcium and magnesium. Add enough agarose to cover the bottom of the petri dish or the well. Pre-warm the plates at RT (18-22 °C). Optionally, stored the plates for couple of days at 4 °C to prevent dehydration.
- Prepare Calcium-free Holtfreter's saline (60 mM NaCl, 0.7 mM KCl, 4.6 mM HEPES, 0.1% BSA (A-7888 Sigma pH 7.6)) and Holtfreter's saline (60 mM NaCl, 0.7 mM KCl, 0.9 mM CaCl2, 4.6 mM HEPES, 0.1% BSA pH 7.6)5. NOTE: the solution of CaCl2 cannot be autoclaved.
- Fill the agarose-coated well to the top with Calcium-free Holtfreter's saline.
- Prepare blastula or very early gastrula embryos to isolate their ACs as previously described (steps 2.5 to 2.7).
- Isolate at least 15 or up to 30, small ACs21. Using fine forceps cut out a small square of tissue out of the animal pole. The AC tissue only contains ectodermal cells and is therefore of uniform thickness. If not, remove the AC from the analysis.
NOTE: The animal pole is the embryo's pigmented part. - Transfer the ACs into an agarose-coated well filled to the top with Calcium-free Holtfreter's saline. Place the ACs with their pigmented side facing upwards.
NOTE: The dissociation process starts rapidly in calcium-free Holtfreter's saline. - Wait a few minutes for cells to start dissociating. Observe this through disaggregation of the tissues. Using fine forceps, separate the pigmented layer from the rest of the AC and discard them with a micropipette P20. Complete cell dissociation process indicates separation of cells from one another.
NOTE: One or two pigmented layers can be left within the re-aggregated explant to help visualization during grafting. - Center the cells using circular movements of the plate. Using a micropipette P1000 carefully remove as much medium as possible. Be careful not to touch the cells.
- Add 1 ml of Holtfreter's saline with calcium to the well. Transfer the dissociated ACs into a 1.5 ml tube.
NOTE: 2 ml tubes cannot be used due to their round bottom. - Pellet the cells by centrifugation, 5 min at a maximum speed of 2,000 rpm for a bench centrifuge (500 x g). Remove carefully the supernatant with a micropipette P1000.
- Add to the dissociated cells 20 µl of Holtfreter's saline with calcium (60 mM NaCl, 0.7 mM KCl, 0.9 mM CaCl2, 4.6 mM HEPES, 0.1% BSA (A-7888 Sigma pH 7.6)5.
- Keep the dissociated ACs, 3 to 6 hr at RT (18-22 °C).
NOTE: This is necessary time for the cells to re-aggregate. The re-aggregation is visible as the cells form a small ball at the bottom of the tube. - Detach the explant carefully from the bottom of the tube by adding 1 ml of 0.5x MBS or of 1 ml of 3/4 NAM (see step 4.1). Transfer the explant in an un-coated plate using a plastic transfer pipette.
- Stage the explants using their control siblings. For long-term culture use antibiotics: kanamycin (50 µg/ml), ampicillin (50 µg/ml) and gentamycine (50 µg/ml).
4. Grafting of Animal Caps Explants in the Neural Plate of X. laevis Embryo
- Prepare 3/4 NAM (110 mM NaCl, 2 mM KCl, 1 mM Ca(NO3)2, 1 mM MgSO4, 0.1 mM EDTA, 1 mM NaHCO3, 0.2x PBS, 50 µg/ml gentamycin). Do not keep for more than 1 week for dissection and store at 4 °C. Older NAM can be used for preparing agarose-coated dissection dishes (below).
- Coat petri dishes (60 mm) with 3% agarose in 3/4 NAM into which small holes have been made using either a silicone mold, or the cover of a table tennis racket.
NOTE: The agarose covers the bottom of the petri dish. Leave some space so that the petri dish to fill with 3/4 NAM. Alternatively make dissection dishes with non-drying modeling clay. Within the clay, squeeze the embryos slightly during the dissection procedure. Dig small holes in the agarose using rounded forceps This 'dissection dish' helps to hold the embryos during the dissection procedure. - Collect dissection tools: plastic transfer pipettes, an 'eyebrow knife' (made with eyebrow hair embedded in paraffin at the tip of a glass Pasteur pipette)22, two fine dissection forceps, P1000 micropipette and tips, a stereosmicroscope with magnification 8-40X, a bright light source with optic fiber guides. Keep all the dissecting tools clean; after each experiment, rinse them twice with distilled water, once with 100% ethanol and let dry. Store away from dust and if necessary autoclave the metal dissecting tools.
- Let the dissociated and re-aggregated ACs (protocol 3), and their siblings X. laevis embryos develop until they reach stage 13 (neurula) according to Nieuwkoop and Faber developmental table17. For transplantation within the neural plate stage 13 to stage 15 embryos are used.
- Carry out all subsequent steps under the stereomicroscope.
- Using a plastic transfer pipette, transfer the embryos into PBS with calcium and magnesium complemented with 0.2% BSA. Remove the embryo's vitelline membrane as previously described20. Fix the embryo with one forceps. Pinch the vitelline membrane with the side of the other forceps. Holding the membrane firmly, slowly peel it off the embryo.
NOTE: Do this procedure on the ventral (vegetal) side of the embryo. Do not damage the embryos neural plate. If embryos have been damaged during the vitelline membrane removal step, transfer the embryos into a 60 mm petri dish containing 3/4 NAM using a plastic transfer pipette and wait 15 min, a sufficient time for the healing process to occur. - Fill in the dissection dish to the top with fresh 3/4 NAM.
- Using a plastic transfer pipette, transfer the embryos without their vitelline membrane into the dissection dish. Place the embryos dorsal side up into the wells. During the transfer process do not allow the embryo to enter in contact with the air-liquid interface since X. laevis are lysed by surface tension.
- Transfer the AC explant to be grafted into the dissection plate using a plastic transfer pipette or a micropipette P1000. Once the pipette is inside the liquid, allow the explants to slowly sink down by gravity, or push very gently.
- Maintain the embryos with rounded forceps. With the eyebrow knife make an incision into the neural plate where you intend to graft your explant's piece.
NOTE: At stage 14 the anterior bending of the neural plate can be use as a landmark, it marks the diencephalon territory (Figure 4). - Cut out a small piece of neuroepithelium, using rounded forceps and an eyebrow knive. Cut a small piece of the explant with the eyebrow knife.
NOTE: The piece of explant should be about the same size and shape as the neuroepithelium ablated area. - Place the piece of explant into the neuroepithelium incision using the eyebrow knife and fine forceps. Ensure that the explant rapidly attaches to the embryo.
- Alternatively place a piece of glass coverslip onto the grafted embryo to maintain the graft in place. To do this, cut a fine glass coverslip into very small pieces using coarse forceps. Ensure that the size is approximately 1.5 mm2. Immerse the pieces into a Petri dish containing 3/4 NAM or PBS using forceps. Choose a piece of glass bigger than the embryo to avoid damaging it. The embryo will be a little bit flattened.
- Wait for at least 30 min without moving, or only move the dissecting plate gently. Let the embryo recover for 30 min to 2 hr and gently remove the coverslip if necessary. Carefully pipette the grafted embryos into a clean dish filled with 3/4 NAM, using the plastic transfer pipette.
NOTE: Grafted embryos tend to develop bacteria or fungi contamination. For long-term culture use antibiotics: kanamycin (50 µg/ml), ampicillin (50 µg/ml) and gentamycine (50 µg/ml).
Representative Results
Based on morphological considerations in different species, embryological manipulations, and the expression pattern of regulatory genes, a conceptual model holds that the neural plate is divided into transverse and longitudinal segments that define a developmental grid generating distinct histogenic fields. In the neural plate, the primordia of the forebrain, midbrain, hindbrain and spinal cord are all already established along the antero-posterior (AP) axis during gastrulation (reviewed in23-25). During neurulation, the histogenic fields can be detected as spatially restricted domains of gene expression. Based on the expression patterns of regulatory genes, the forebrain primordium is divided into six transverse segments that generate distinct histogenic fields called prosomeres (p). Each prosomere is divided into a ventral (basal) and a dorsal (alar) part (Reviewed in26,27). In amphibians, the prosomere 2 (p2) gives rise to the epithalamus and the thalamus and to its organizer the Zona Limitans Intrathalamica (ZLI)8 (reviewed in28,29).
On Figure 1 are shown the flat-mounting of X. laevis and X. tropicalis anterior neural tubes at stages 30, 32, and 35 after WM-DISH. WM-ISH were performed according to protocol 1 using probes for the transcription factors fezf2 that marks the telencephalon and p3, barhl2 that marks p2, the cortical hems and the midbrain, iroquois-1(irx1) and iroquois-3 (irx3) that respectively mark caudal p2 and alar p2 and for the morphogen shh that marks the ZLI, the basal forebrain and midbrain (Figure 1E). A comparative analysis of the various gene expression patterns reveals that the anterior limit of barhl2 p2 expression abuts the caudal limit of fezf2 expression in p3 (Figure 1A). irx3 is co-expressed with shh in the future ZLI (Figure 1B). Inside p2 irx1 expression domain is complementary to that of shh (Figure 1C)8. The expression pattern of barhl2 in X. tropicalis at stage 35 is shown in Figure 1D. A schematic diagram of the expression territories of these genes in the developing amphibian forebrain is provided in Figure 1E.
Using protocol 2 the ability of anion exchange resin beads soaked in Shh to induce shh expression was investigated in ACs explants. X. laevis embryos were injected into the 4 animal blastomeres at the 4 and 8-cells stage with mRNAs encoding for the anterior neuroepithelial inducer noggin together with barhl2, otx2,irx3 and gfp as an injection tracer. The expression of the anti-BMP factor Noggin inhibits the BMP pathway and gives a neural identity to AC cells3. We refer to AC expressing Noggin as Anteriorised Animal Cap (AAC). Blastocoel roof explants were prepared as described in Protocol 2. The AACs were cultured for 48 hr at 18 °C in contact with an anion exchange resin bead soaked in a conditioned medium (CM) containing, or not, the secreted N-terminal part of the morphogen sonic hedgehog from rat (N-Shh). The expression of endogenous shh was analysed by ISH (Figure 2). Expression of Xenopus shh (Xshh) is detected in cells in contact with the bead soaked in CM with N-Shh but not in cells in contact with the bead soaked in CM without N-Shh (Figure 2B).
Using protocol 3 the ability of different cell types to segregate from one another was investigated. X. laevis embryos were injected into the 4 animal blastomeres at the 4 and 8-cells stage with mRNAs encoding for noggin with or without otx2, barhl2 and irx3, as indicated, using ßGal or Gfp as tracers (Figure 3A). Stage 8/9 ACs were dissociated and re-aggregated to generate explants composed of mixed neuroepithelial cells containing both ßGal- and Gfp-expressing cells. The reaggregated explants were cultured for 48 hr at 18 °C. ßGal activity was revealed in red according to30 (Figure 3A). The behavior of cells was observed by following their localization within the re-aggregated explants. In explants where AACs cells are mixed with AACs cells expressing Otx2 (red), the ßGal-expressing cells do not segregate from the Gfp-expressing cells both types of cells intermingled freely (Figure 3B, 3C). In contrast, in explants where Otx2-expressing cells (Gfp) are mixed with Barhl2, Otx2, Irx3 (BOI3)-expressing cells (ßGal), the ßGal-expressing cells (red) form cohesive patches and do not spread uniformly within the re-aggregated explant (Figure 3D, 3E).
Using Protocol 4, the behavior of programmed AC cells was investigated in vivo. X. laevis embryos were injected into the 4 animal blastomeres at the 4 and 8-cell stages with mRNAs encoding for noggin with otx2 eitheralone or together with barhl2 and irx3. ßGal was used as tracer. In parallel, embryos were similarly injected with mRNAs encoding for noggin and the secreted N-terminal part of the morphogen shh from rat (N-shh). At stage 8/9, AC explants were dissociated and immediately re-aggregated to generate mixed explants composed of neuroepithelial cells either expressing N-Shh and Otx2, or expressing N-Shh and Otx2, Barhl2 and Irx3 (BOI3). Small pieces of the mixed explants were grafted into the neural plate of X. laevis embryos at stage 14. The grafted cells were marked with ßGal staining (red). At stage 35 the expression of endogenous shh was analyzed by ISH. When grafted into the anterior neural plate of X. laevis embryos, mixed explants composed of BOI3 and N-Shh expressing cells developed two features specific of ZLI cells: the grafted cells expressed Xshh and segregated from anterior neuroepithelial cells (Figure 3C). In contrast, when mixed explants composed of Otx2– and N-Shh- expressing cells are grafted in the X. laevis neural plate, the grafted cells did not express shh and did not segregate from their neighbors (Figure 3B)8.
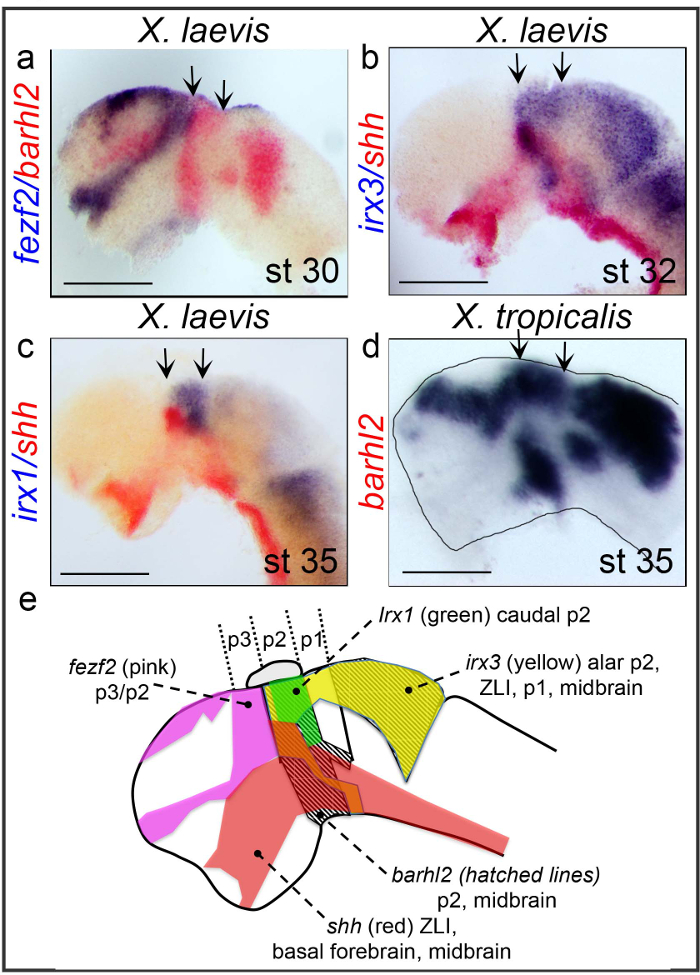
Figure 1. Flat-mounted neural tubes from X. laevis and X. tropicalis embryos whole-mount double ISH. (A–D) WM-ISH is performed using fezf2, barhl2, irx1, irx3, and shh as probes on (A–C) X. laevis embryos at stages 30, 32, and 35 and (D) X. tropicalis embryo at stage 35 as indicated. The neural tubes of representative embryos are dissected and flat mounted as described in protocol 1. The dissected neural tubes are shown from a side view, dorsal up, anterior left. The markers and stages are indicated. The rostral and caudal boundaries of p2 are indicated with arrows. (A-C) The scale bar stands for 0.5 mm. (D) The scale bar stands for 0.1 mm. (E) Schematic of forebrain markers at st.30. barhl2 expression domain is indicated in hatched lines; Areas of expression are shown for fezf2 (pink), shh (red), irx3 (yellow) and irx1 (green). p stands for prosomere. The pineal gland located on top of p2 is shown. Please click here to view a larger version of this figure.
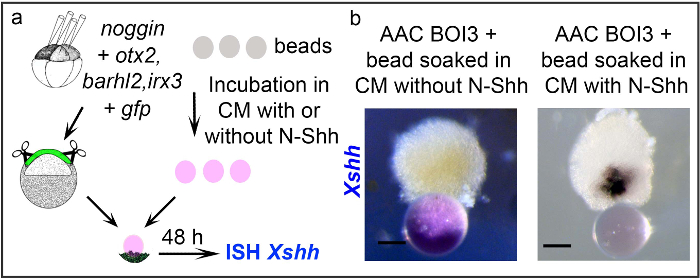
Figure 2. Induction of shh expression in programmed explants via contact with anion exchange resin beads. Small ACs are prepared from embryos injected with mRNA encoding for noggin, barhl2, otx2, and irx3, with Gfp as tracer. AAC stands for Anteriorised Animal Cap. The AAC explants expressing Barhl2, Otx2 and Irx3 (AAC BOI3) are cultured for 48 hr in contact with a bead soaked in a conditioned medium (CM) either containing N-Shh, or not, as indicated. The explants are analyzed by ISH for the expression of Xenopus (X) shh. The scale bar stands for 0.5 mm. Please click here to view a larger version of this figure.
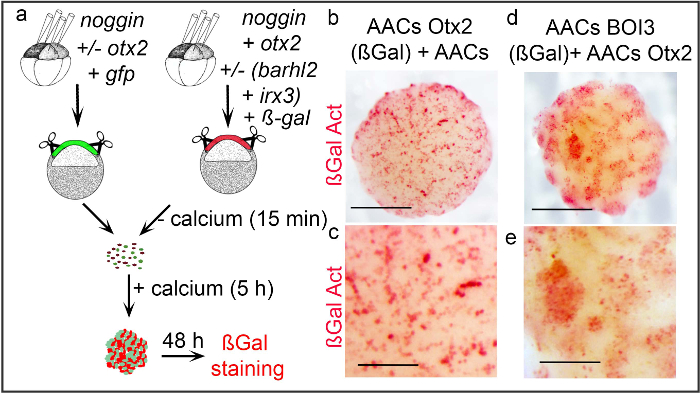
Figure 3. Analysis of cell segregation behavior in re-aggregated explants. (A) X. laevis embryos are injected with mRNA encoding for noggin, otx2, barhl2 and irx3 as indicated. ßGal and Gfp are used as tracer. At stage 8/9 small ACs are prepared, dissociated and re-aggregated to generate explants made of Gfp/ßGal mixed cell types. The explants are cultured for 48 hr at 18 °C and ßGal activity is revealed (red). (B–E) Representative explants composed of mixed cells expressing proteins as indicated are shown. AAC stands for Anteriorised Animal Cap. (B, C) ACs cells expressing Noggin and Otx2 (ßGal) spread randomly when mixed with ACs cells expressing Noggin (Gfp). (C) Enlarged view of (B). (D) AACs cells expressing Barhl2, Otx2 and Irx3 (BOI3) (ßGal) regrouped and segregated from AACs cells expressing Otx2 (Gfp). (E) Enlarged view of (D). (B, D) The scale bar stands for 0.5 mm. (C, E) The scale bar stands for 0.2 mm. Please click here to view a larger version of this figure.
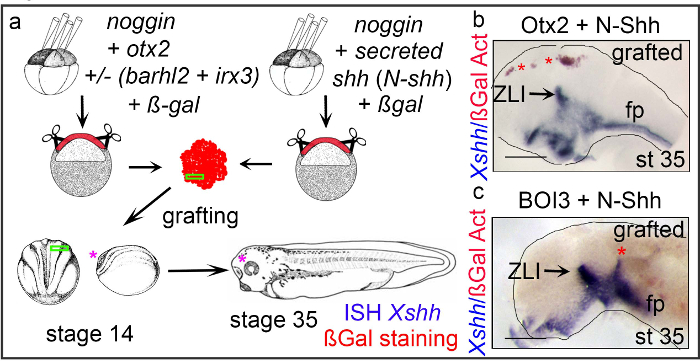
Figure 4. Programmed explants exhibit their development features when grafted in the neural plate of a stage 14 embryo. (A) Experimental scheme: AACs are prepared from embryos injected with mRNAs as indicated. At stage 8/9 cells from the roof of the blastocoel are dissociated and re-aggregated to generate explants made of mixed cell types expressing ßGal (red). The explants are cultured until their siblings reached stage 14. A small piece of mixed explant (green rectangle) is put into an incision within the anterior neural plate. Operated embryos are grown until stage 35. Operated embryos are analyzed by WM-ISH using X. laevisshh (Xshh) as probe. ßGal activity is revealed in red according to30. (B, C) The grafted sides of stage 35 representative neural tubes are shown, side view, dorsal up, anterior left. (B) Grafted with mixed AACs expressing Otx2 and N-Shh. (C) Grafted with mixed AACs expressing Barhl2, Otx2, Irx3 (BOI3) and N-Shh. Grafted cells are indicated with a red star. The pink star indicates the prospective diencephalon. Please click here to view a larger version of this figure.
Discussion
Neural development is orchestrated by a complex interplay between cellular developmental programs and signals from the surrounding tissues (Reviewed in3,31,32). Here we describe a set of protocols that can be used in X. laevis embryos to explore extrinsic and intrinsic factors involved in neural fate determination and neural morphogenesis in vitro and in vivo. These protocols can be used as such on X. tropicalis embryos, however X. tropicalis embryos are four times smaller then X. laevis embryos. Both the forceps and the tungsten needles used need to be finer. When possible, use X. laevis.
Precise visualization of X. laevis and X. tropicalis neural histogenic fields can be achieved using the histological flat mounting of dissected neural tubes from WM- ISH. This technique was performed successfully on X. laevis embryos from stage 26 to stage 45 (Figure 1) and on X. tropicalis embryos from stage 30 to stage 458,33. Older embryos are easier to dissect then younger embryos. This technique is easy to perform. It permits analysis of gene expression patterns, separately or combined, on neural tubes at different developmental stages. With this protocol it is easy to compare one side of a neural tube with the other. This is very useful in Xenopus GOF and LOF experiments. The defects observed on the injected side of the neural tube can be directly compared with normal developmental events observed on the control side. This strategy has been used to analyze GOF and LOF phenotypes in X. laevis embryos8,33. One important step in this protocol is the correct fixation of the embryonic tissues. An incomplete fixation prevents the efficient detachment of the ectoderm from the neural tube. The pre-dissection of the neural tube facilitates the penetration of in situ probes. However once isolated, it is easy to lose the neural tubes. It is advisable to keep part of the embryo during the ISH procedure to prevent any loss.
The embryos used for all these experiments need to be robust and need to heal well. Discard any batch of unhealthy embryos. In order to dissect ACs and graft explants between stage 13 and stage 15 it is possible to grow X. laevis explants and embryos at various temperatures (from 12-18 °C and 18-20 °C). Noteworthy X. laevis embryonic cells contain enough yolk to allow their survival for several days without addition of any external nutrient. To not damage the embryonic tissues, any transfer procedure of embryo, AC, or explant has to be done carefully. It is advisable to use either a plastic transfer pipette or micropipette if necessary with a tip cut at its end. The diameter of the tip end is adjusted to the size of the embryo, or the size of explant, to be transferred. It is worthy of note, coating pipettes with a 0.1% BSA solution prevents the adhesion of small pieces of tissue to the plastic.
AC explants can be programmed through forced gene expression, or through exposure to extrinsic factors in a time- and space-controlled manner. We describe a technique allowing local induction via direct contact between an explant and a bead. If necessary the beads can be further fragmented using forceps. Compared to other previously described strategies, this technique allows the testing of local induction with pure factors, alone or in combination, in a precise time window.
Using a previously described approach of cell dissociation and re-association34, it is possible to investigate cell motility capacities, cell segregation behaviors, and formation of compartment boundaries in sandwiched and mixed AC explants8. The cell dissociation process starts in less than 15 min. Avoid shaking the plate during the dissociation step to prevent the loss of cells. The AC pigmented layers do not dissociate well. It is recommended to remove them during the dissociation step. A couple of AC pigmented layers are left in the re-aggregated explant. It helps in visualizing the graft. It does not interfere with analysis at later developmental stages.
In this manuscript a protocol to transplant mixed explants into a neural plate is detailed. In Xenopus embryos, the neural plate is open between stage 13 and stage 15. These stages are therefore appropriate for transplantation within the neuroepithelium. X. laevis tissues adhesive properties change with time. It is important to graft AC explants into a host at a similar developmental stage. At early developmental stages, X. laevis embryos grown in 3/4 NAM repair extremely fast. If some embryos have been damaged during removal of the vitelline membrane it may be useful to wait 15 min, which is the time necessary for the healing process to occur. Insertion can be favored by putting a piece of glass coverslip onto the grafted host tissue, but it is not a requirement. The force generated by the healing process can extrude the grafted cells. Morphology of the host embryo is better preserved when the graft is forced into it with external pressure, as with a coverslip. To assess the success of the graft, a fluorescent tracer can be injected into the explants. The presence of fluorescent cells within the grafted embryo can be visualized until the neural tube closes. The major drawback in AC culture and grafting experiments is the frequency of bacteria or fungi contamination. Use clean or sterile material and sterile solutions as often as possible to avoid dish contamination at every step of the protocol. For long term culture of grafted embryos always use antibiotics and a temperature of 15 °C. Avoid long-term contact of AC explants or embryos with agarose. We observed that it can decrease the life expectancy of the explant. The grafting technique is powerful but time consuming. It is not advisable to screen for fate determination cues with it, but to acquire enough information on your tissue of choice beforehand.
Beside its traditional advantages, the recent genetic advances in Xenopus opened the way for this model system to explore gene regulatory networks using large-scale genomic analysis by deep RNA sequencing and Chromatide Immunoprecipitation-sequencing (ChIP-seq)5,35. There are excellent RNAseq and Chip-Seq reference data sets covering early embryonic development. One of the ChIP-seq analysis drawbacks is the need to collect large amount of material. Programming of AC explants permits production of a large amount of a specific neural tissue, and at least partly, helps to overcome this technical limit.
The developmental programs underlying neural tube organogenesis are largely conserved, especially in vertebrates. Information acquired using amphibians helps in the understanding of cellular and molecular processes underlying vertebrate development25,32,36-38. Recent progress in the field of Induced Pluripotent Stem Cells (iPSCs) has opened up gateways for the research in regenerative medicine and drug discovery. iPSCs are programmed from somatic cells using a combination of transcription factors. The search of programming cues for stem cells is ongoing. The assays describe here provide a mean to generate neural-derived cell types in vitro that could be used in drug screening. They also provide a cheap and fast way to test for neural fate determinants that can be used for further reprogramming of iPSCs39,40.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The author thanks Hugo Juraver-Geslin, Marion Wassef and Anne Hélène Monsoro-Burq for their help and advice, and the Animal Facility of the Institut Curie. The author thanks Paul Johnson for his editing work on the manuscript. This work was supported by the Centre National de la Recherche Scientifique (CNRS UMR8197, INSERM U1024) and by grants from the “Association pour la Recherche sur le Cancer” (ARC 4972 and ARC 5115; FRC DOC20120605233 and LABEX Memolife) and the Fondation Pierre Gilles de Gennes (FPGG0039).
Materials
| Paraformaldehyde | VWR | 20909.290 | Toxic |
| anion exchange resin beads | Biorad | 140- 1231 | |
| Bovine Serum Albumin | SIGMA | A-7888 | For culture of animal cappH 7.6 |
| Gentamycine | GIBCO | 15751-045 | antibiotic |
| Bovine Serum Albumin | SIGMA | A7906 | for bead preparation |
References
- Nieuwkoop, P. D. IIB, Pattern formation in the developing central nervous system (CNS) of the amphibians and birds (English). Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen. 94, 121-127 (1991).
- Nieuwkoop, P. D. The neural induction process; its morphogenetic aspects. Int J Dev Biol. 43, 615-623 (1999).
- Harland, R. Neural induction. Curr Opin Genet Dev. 10, 357-362 (2000).
- Sive, H. L., Grainger, R. M., Harland, R. M. . Early development of Xenopus laevis : a laboratory manual. , (2000).
- Hoppler, S., Vize, P. D., Hoppler, S., Vize, P. D. . Xenopus protocols : post-genomic approaches. , (2012).
- Franklin Hughes, W., La Velle, A. The effects of early tectal lesions on development in the retinal gonglion cell layer of chick embryos. J Comp Neurol. 163, 265-283 (1975).
- Theveneau, E., Mayor, R. Beads on the run: beads as alternative tools for chemotaxis assays. Methods Mol Biol. 769, 449-460 (2011).
- Juraver-Geslin, H. A., Gomez-Skarmeta, J. L., Durand, B. C. The conserved barH-like homeobox-2 gene barhl2 acts downstream of orthodentricle-2 and together with iroquois-3 in establishment of the caudal forebrain signaling center induced by Sonic Hedgehog. Dev Biol. 396, 107-120 (2014).
- Green, J. B., New, H. V., Smith, J. C. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 71, 731-739 (1992).
- Wallingford, J. B., Ewald, A. J., Harland, R. M., Fraser, S. E. Calcium signaling during convergent extension in Xenopus. Curr Biol. 11, 652-661 (2001).
- Kiecker, C., Niehrs, C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. DEVELOPMENT. 128, 4189-4201 (2001).
- Wilson, P. A., Hemmati-Brivanlou, A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 376, 331-333 (1995).
- Afouda, B. A., Hoppler, S. Xenopus explants as an experimental model system for studying heart development. Trends in cardiovascular medicine. 19, 220-226 (2009).
- Afouda, B. A. Stem-cell-like embryonic explants to study cardiac development. Methods Mol Biol. 917, 515-523 (2012).
- Milet, C., Monsoro-Burq, A. H. Dissection of Xenopus laevis neural crest for in vitro explant culture or in vivo transplantation. Journal of visualized experiments: JoVE. , (2014).
- Milet, C., Maczkowiak, F., Roche, D. D., Monsoro-Burq, A. H. Pax3 and Zic1 drive induction and differentiation of multipotent, migratory, and functional neural crest in Xenopus embryos. Proc Natl Acad Sci U S A. 110, 5528-5533 (2013).
- Nieuwkoop, P. D., Faber, J., Nieuwkoop, P. D., Faber, J. . Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. , (1994).
- Harland, R. M. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 36, 685-695 (1991).
- Turner, D. L., Weintraub, H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 8, 1434-1447 (1994).
- Sive, H. L., Grainger, R. M., Harland, R. M. Removing the Vitelline Membrane from Xenopus laevis Embryos. CSH protocols. , (2007).
- Sive, H. L., Grainger, R. M., Harland, R. M. Animal Cap Isolation from Xenopus laevis. CSH protocols. , (2007).
- Sive, H. L., Grainger, R. M., Harland, R. M. Embryo dissection and micromanipulation tools. CSH protocols. , (2007).
- Wilson, S. W., Houart, C. Review: Early Steps in the Development of the Forebrain. Developmental Cell. 6, 167-181 (2004).
- Juraver-Geslin, H. A., Durand, B. C. Early development of the neural plate: new roles for apoptosis and for one of its main effectors caspase-3. Genesis. 53, 203-224 (2015).
- Heasman, J. Patterning the early Xenopus embryo. Development. 133, 1205-1217 (2006).
- Rubenstein, J. L., Martinez, S., Shimamura, K., Puelles, L. The embryonic vertebrate forebrain: the prosomeric model. Science. 266, 578-580 (1994).
- Puelles, L., Rubenstein, J. L. R. Forebrain gene expression domains and the evolving prosomeric model. Trends in Neurosciences. 26, 469-476 (2003).
- Martinez-Ferre, A., Martinez, S. Molecular regionalization of the diencephalon. Frontiers In Neuroscience. 6, 73-73 (2012).
- Scholpp, S., Lumsden, A. Review: Building a bridal chamber: development of the thalamus. Trends in Neurosciences. 33, 373-380 (2010).
- Coffman, C., Harris, W., Kintner, C. Xotch, the Xenopus homolog of Drosophila notch. Science. 249, 1438-1441 (1990).
- Pera, E. M., Acosta, H., Gouignard, N., Climent, M., Arregi, I. Active signals, gradient formation and regional specificity in neural induction. Exp Cell Res. 321, 25-31 (2014).
- Stern, C. D. Neural induction: old problem, new findings, yet more questions. Development. 132, 2007-2021 (2005).
- Juraver-Geslin, H. A., Ausseil, J. J., Wassef, M., Durand, B. C. Barhl2 limits growth of the diencephalic primordium through Caspase3 inhibition of beta-catenin activation. Proc Natl Acad Sci U S A. 108, 2288-2293 (2011).
- Sive, H. L., Grainger, R. M., Harland, R. M. Dissociation and Reaggregation of Xenopus laevis Animal Caps. CSH protocols. , (2007).
- Harland, R. M., Grainger, R. M. Xenopus research: metamorphosed by genetics and genomics. Trends Genet. 27, 507-515 (2011).
- Beccari, L., Marco-Ferreres, R., Bovolenta, P. The logic of gene regulatory networks in early vertebrate forebrain patterning. Mech Dev. 130, 95-111 (2013).
- Pani, A. M., et al. Ancient deuterostome origins of vertebrate brain signalling centres. Nature. 483, 289-294 (2012).
- Holland, L. Z., et al. Evolution of bilaterian central nervous systems: a single origin?. Evodevo. 4, 27 (2013).
- Pratt, K. G., Khakhalin, A. S. Modeling human neurodevelopmental disorders in the Xenopus tadpole: from mechanisms to therapeutic targets. Disease models & mechanisms. 6, 1057-1065 (2013).
- Sasai, Y., Ogushi, M., Nagase, T., Ando, S. Bridging the gap from frog research to human therapy: a tale of neural differentiation in Xenopus animal caps and human pluripotent cells. Development, growth & differentiation. 50, s47-s55 (2008).

