Isolation and Decellularization of a Whole Porcine Pancreas
Summary
Tissue engineering of the whole pancreas is a challenge because of its exocrine and endocrine functions. We show a method for the dissection of an intact porcine pancreas and the process of successful decellularization by perfusion of detergents Triton X-100, sodium deoxycholate, and deoxyribonuclease.
Abstract
Tissue engineering of the whole pancreas can improve current treatments for diabetes mellitus. The ultimate goal is to tissue engineer pancreas from an allogeneic or xenogeneic source with human cells. A demonstration of methods for the efficient dissection, decellularization, and recellularization of porcine pancreas might benefit the field. Akin to human pancreases, porcine pancreases have a special anatomical arrangement with three lobes (splenic, duodenal, and connection) rounded by the duodenum and small intestine. The duodenal lobe of the pancreas connects to the duodenum by several small blood vessels. Tissue engineering of the pancreas is complicated because of its exocrine and endocrine nature. In this paper, we show a detailed protocol to dissect the whole porcine pancreas and decellularize it with detergents while saving its structure and some extracellular matrix components. To achieve complete perfusion, the aorta is chosen as inlet and the portal vein as outlet. The other blood vessels (hepatic artery, splenic vein, splenic artery, mesenteric artery and vein tree) and bile duct are ligated. To prevent the formation of thrombus, the pig is heparinized and, immediately after dissection, the organ is flushed with cold heparin. To inhibit the action of exocrine enzymes, the pancreas decellularization is set at 4 °C. The decellularization is performed by perfusion of Triton X-100, sodium deoxycholate, and deoxyribonuclease, with an intermittent and final extensive washing. With a successful decellularization, the pancreas appears white, and a histological evaluation with hematoxylin and eosin shows an absence of nuclei with a preserved extracellular matrix structure. Thus, the proposed method can be used to successfully dissect and decellularize whole porcine pancreas.
Introduction
Diabetes mellitus is characterized by the presence of increased levels of glucose in blood. It is recognized as a major public health challenge in most countries1. High levels of blood glucose affect the blood vessels and nervous system, causing damage to the eyes, the heart, and the kidneys, and extremity ischemia. Traditional methods of treatment include injections of exogenous insulin, drugs, and lifestyle changes. Putting aside a cure for the disease, in some cases, available treatments fail to maintain the insulin at therapeutic levels, resulting in hyperglycemia. Although the transplantation of islets or whole pancreas eliminates the disease, it is not commonly done because of a shortage of suitable donor organs and because of the risks and difficulties involved from immunosuppression and encapsulation2.
Current improvements in the field of tissue engineering and regenerative medicine possess the capacity for providing a solution for these issues. With the technique of decellularization, the cellular material from a human or animal donor can be removed while the important extracellular matrix (ECM) proteins, growth factors, and signaling molecules are preserved in the scaffold. Such scaffolds can potentially be transplanted without the need for immunosuppression, to restore the organ function after recellularization with the recipient's own non-immunogenic stem cells3,4. The tissue-engineered organs from allogeneic or xenogeneic sources can be used in clinical transplantation, as the major extracellular matrix proteins are conserved among species and might not be rejected after transplantation5.
Decellularization is a well-explored method involving the optimal use of physical forces, chemical detergents, and enzymes in a physiological setting to remove cells and nuclear material from a tissue or organ. Recellularization is a procedure of seeding cells back into the acellular organ. It is an intellectually tough procedure, requiring a large number of cells, an optimum cell-seeding strategy, and a bioreactor system for the culture of the organ at physiologically acceptable conditions like temperature, pressure, and gases6.
The pancreas can be considered a challenging tissue for tissue engineering because of its exocrine and endocrine capacities. The exocrine tissue secretes several digestive enzymes, while the endocrine part secretes hormones, including insulin. The decellularization of intact pancreases from mouse7,8, human9, and pig10 has already been reported using enzymes (trypsin, deoxyribonuclease [DNase]) and non-ionic (Triton X-100) and ionic detergents (sodium deoxycholate [SDC] and sodium dodecyl sulfate [SDS]). However, following the published protocols, we struggled with a successful dissection and complete perfusion and decellularization while maintaining an ECM structure. We speculated that the applied detergents during the decellularization cause lysis of the cells, thereby releasing digestive enzymes into the organ. The released enzymes will cause an irreversible damage to the ECM scaffold and make it inefficient for decellularization and recellularization. A design of the method that effectively decellularizes pancreas while inhibiting the action of digestive enzymes may solve the problem. We chose the strategy of Peloso et al., of decellularization of the pancreas at a cold temperature, although they did not report on why cold temperature is used9. At the same time, we designed a dissection strategy with modifications from Taylor et al. by choosing the aorta as a perfusion inlet over the coeliac trunk (CT) and the superior mesenteric artery (SMA)11.
In a recently published article12, we demonstrate a method for the effective isolation and decellularization of porcine pancreas while preserving some ECM components. In this paper, we show a detailed description of how to dissect a whole porcine pancreas containing splenic, duodenal, and connection lobes, and present a stepwise protocol for successful decellularization.
Protocol
The dissection of a porcine pancreas and the decellularization procedure presented here follow the ethical guidelines of the University of Gothenburg.
1. Preparation of the Decellularization Set-up
- Using 3 x 5 mm silicone tubes, connect in series the detergent inlet container to the peristalic pump and then to the pancreas in the organ chamber via the degasser (see Figure 1). Connect a male luer to the free end of tube in organ chamber.
- Using another 3 x 5 mm silicon tube, connect the organ chamber to the detergent outlet container via the peristaltic pump to collect the perfused detergent.
- Connect a 2 ml unlabeled pipette to the free ends of tubes in detergent inlet container and detergent outlet cointainer.
- Keep the whole set-up at 4 °C.

Figure 1: Preparation of the perfusion set-up. Using a 3 x 5 mm silicone tube, as shown in the set-up, connect in series the detergent inlet container to the peristaltic pump, the degasser and the organ chamber. The black arrows show the flow direction from detergent inlet container to organ chamber. For the detergent outlet, use another 3 x 5 mm silicone tube and connect the organ chamber via the peristaltic pump to the detergent outlet container. The red arrows show the flow direction from organ chamber to detergent outlet container. Please click here to view a larger version of this figure.
2. Preparation of Decellularization Solutions
- Solution 1 (phosphate-buffered saline [PBS]): Add 8 g of (137 mM) sodium chloride, 0.2 g of (2.7 mM) potassium chloride, 1.44 g of (10 mM) sodium phosphate, and 0.24 g of (1.8 mM) potassium phosphate to 1 L of ultrapure water and stir until dissolved. Adjust the pH to 7.4 with hydrochloric acid (HCl). In total, 3.2 L of this solution is required.
- Solution 2 (PBS + heparin): To 1 L of Solution 1, add 3.4 mL of heparin (17 international units [IU]/mL). Prepare this solution fresh and keep it on ice until it becomes cold. In total, 1.2 L of this solution is required.
- Solution 3 (ultrapure water + sodium azide + disodium ethylenediaminetetraacetic acid [EDTA]): To 1 L of ultrapure water, add 1.86 g of (5 mM) EDTA and 200 mg of (0.02%) sodium azide. Stir until the salts are dissolved. Cool the solution to 4 °C before use. In total, 22 L of this solution is required.
- Solution 4 (PBS + sodium azide + EDTA): To 1 L of Solution 1, add 200 mg of (0.02%) sodium azide and 1.86 g of (5 mM) EDTA. Stir until the salts are dissolved. Cool the solution to 4 °C before use. In total, 1 L of this solution is required.
- Solution 5 (ultrapure water + sodium azide): To 1 L of ultrapure water, add 200 mg of (0.02%) sodium azide. Stir until the salts are dissolved. Cool the solution to 4 °C before use. In total, 260 L of this solution is required.
NOTE: EDTA forms a precipitate with SDC and was excluded from Solution 5 since it will be used for immediate washing before and after the SDC treatment. - Solution 6 (SDC + Triton X-100): To 940 mL of ultrapure water, add 40 g of (4%) SDC, 60 mL of (6%) Triton X-100, 200 mg of (0.02%) sodium azide, and 69.6 mg of (0.4 mM) phenylmethylsulfonyl fluoride (PMSF). Stir until dissolved. Cool the solution to 4 °C before use. Add PMSF before use. In total, 9.6 L of this solution is required.
- Solution 7 (DNase): Add 10,000 Kunitz units of DNase-I in 250 mL of (40 Kunitz units/mL) Dulbecco's PBS containing CaCl2 and MgCl2. Prepare fresh and use immediately. Warm the solution to 37 °C before use.
- Solution 8 (PBS + sodium azide): To 1 L of Solution 1, add 200 mg of (0.02%) sodium azide. Stir until the salts are dissolved. Cool the solution to 4 °C before use. In total, 1 L of this solution is required.
3. Dissection of the Porcine Pancreas
NOTE: In this study, porcine pancreases were dissected from euthanized, heparinized (400 IU/kg) female pigs weighing 45 kg from a farm.
- Place the pig on the dissection table in the supine position.
- Make a midline incision from the xiphoidal process to the pubic bone (approximately 40 cm) using a scalpel, exposing all abdominal organs.
- Locate the splenic, duodenal, and connection lobes.
- Locate the level of the major duodenal papilla and ligate the duodenum orally from that site using two separate sutures.
- Ligate the inferior esophagus with two separate sutures and cut between the ligatures with scissors to take the stomach out.
- Separate the connective tissue from the colon to reach the small intestine.
- Separate the connective tissue of the colon that attaches to the splenic lobe of the pancreas.
- Remove the colon from the small intestine after ligating the arteries.
- Find the inferior mesenteric vein and the inferior mesenteric artery tree and ligate with one suture where they appear caudally of the pancreas.
- Ligate the splenic artery and vein together with one suture, close to the spleen, in the hilum, and cut distally with scissors to remove the spleen.
- Follow the duodenum until the duodenal and connection lobes are cleared and ligate the duodenum at the end with two separate sutures.
- Dissect the portal vein, ligate it with one suture to prevent any blood leakage from the liver and cut proximally to ligature. The portal vein serves as an outlet during the decellularization.
- Dissect and ligate the bile duct and hepatic artery with two sutures. Cut distally to sutures.
- Find the aorta under the renal vein and dissect it in the cranial direction from muscle and connective tissue until it reaches the pancreatic area.
- Flip the pancreas gently over and dissect the aorta, keeping the SMA and the CT intact, and cut the aorta superior to CT and inferior to SMA with scissors.
- Cut the remaining surrounding tissues with scissors and take the pancreas out.
- Using a 50-mL syringe connected to 4-mm arteriotomy cannula, flush the organ through the aorta with Solution 2 until it perfuses the whole organ or until the whole organ becomes cold.
4. Preparation of the Porcine Pancreas for Decellularization
- Keep the pancreas at 4 °C or on ice throughout the process.
- Cut the sutures of the duodenum using scissors, clean it of food by flushing 50 – 150 mL of ultrapure water using a 25-mL pipette, and ligate it again with sutures.
- Ligate one end of the aorta and all branches besides the SMA and CT with sutures to prevent leakage.
- Insert from the other end of the aorta a 4-mm arteriotomy cannula and ligate it with sutures.
- Perfuse the pancreas with Solution 3 for 1 h at 20 mL/min using the decellularization set-up.
NOTE: Prefill the tubes of pump with solution 3 such that no bubbles enter the pancreas. Look for any leakages from all sides of the organ and ligate all open vascular branches with sutures, except the portal vein. - Freeze the pancreas at -20 °C in Solution 4 until the start of the decellularization.
5. Decellularization of the Porcine Pancreas
- Thaw the pancreas at 4 °C.
- Run the peristaltic pump in the decellularization set-up with Solution 3 at 20 mL/min until no air bubbles are seen in the detergent inlet tube.
- Place the pancreas in the decellularization container and connect the detergent inlet tube to the aorta of the pancreas. Wash the organ by perfusion with Solution 3 overnight at 20 mL/min at 4 °C.
- Pour out the solution left in the organ chamber.
- Replace Solution 3 with Solution 5 and perfuse the pancreas for 30 min at 20 mL/min at 4 °C. Pour out the solution in the organ chamber.
- Add Solution 6 and perfuse the pancreas for 8 h at 20 mL/min at 4 °C. Pour out the solution in the organ chamber.
- Wash the organ by perfusion with Solution 5 for 96 h at 20 mL/min at 4 °C. Pour out the solution in the organ chamber.
- Prepare the pancreas for DNase treatment by recirculating 500 mL of Dulbecco's PBS containing CaCl2 and MgCl2 for 30 min at 37 °C. Pour out the solution in the organ chamber.
- Add 250 mL of Solution 7 and perfuse the pancreas for 4 h at 20 mL/min at 37 °C. Pour out the solution in the organ chamber.
- Wash the organ by perfusion with Solution 5 for 120 h at 20 mL/min at 4 °C.
- Store the organ in Solution 8 at 4 °C for short periods or at -20 °C for long periods.
6. Verification of Decellularization
- Using scissors, cut 3- to 10-mm biopsies from all lobes of the pancreas and fix them in formaldehyde for 48 h at room temperature.
- Wash the pieces in ultrapure water for 15 min, process them in a tissue processor following standard protocols, and embed them in paraffin.
- Cut 5-µm sections using microtome and stain them by Meyer's hematoxylin and 0.2% alcoholic eosin (HE) following standard protocols.
- View the slides under a light microscope to check for the loss of nuclei.
NOTE: A piece from a fresh tissue processed in the same way can be used as a control to check for the presence of nuclei.
Representative Results
Representative porcine pancreas dissection pictures, which can help in locating and dissecting the inferior mesenteric artery and vein tree, the portal vein, the hepatic artery, the bile duct, and the aorta branching to CT and SMA, are shown in Figure 2A, 2B, and 2C (yellow arrows), respectively. Figure 3A shows the gross morphology of a normal pancreas, which appears light pink and contains splenic, connection, and duodenal lobes. After decellularization, the pink color is lost and the decellularized pancreas looks pale white in color. The gross morphology picture showing splenic, connection, and duodenal lobes of a decellularized pancreas is shown in Figure 3B. Figure 3C shows the presence of many blue nuclei in a normal pancreas by staining with HE. In a decellularized pancreas, the HE staining showed a loss of nuclei, as no blue nuclei are seen (Figure 3D).
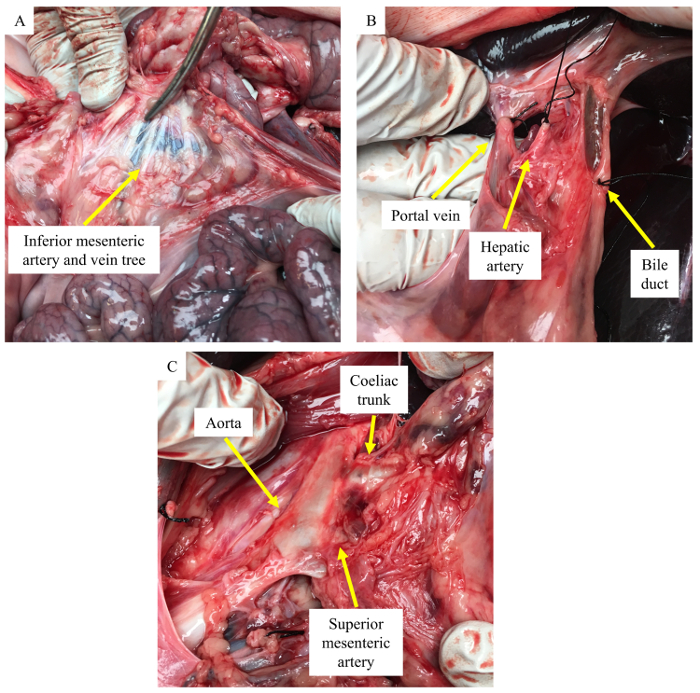
Figure 2: Pictures of a porcine pancreas dissection. (A) Location of the inferior mesenteric artery and vein tree (yellow arrow). (B) Ligation of the portal vein, the hepatic artery and the bile duct (yellow arrow). (C) Aorta branching to the coeliac trunk and the superior mesenteric artery (yellow arrow). Please click here to view a larger version of this figure.
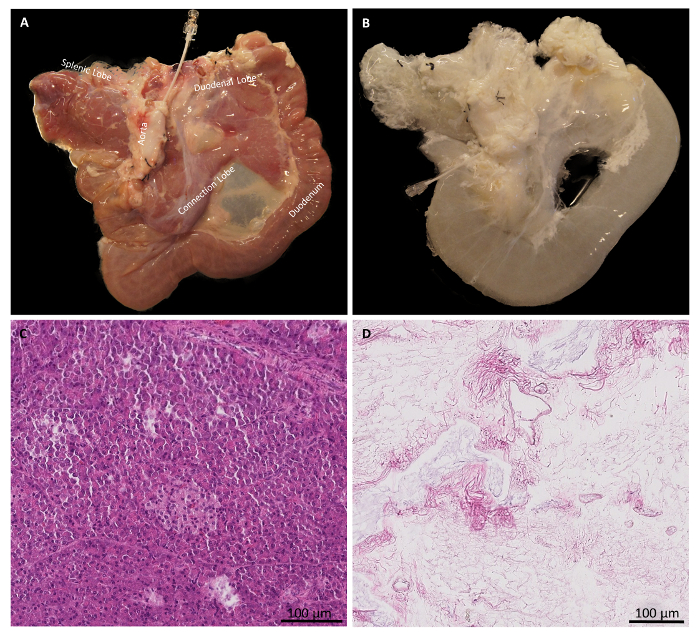
Figure 3: Gross morphology and HE staining of normal and decellularized pancreases. (A) Gross morphology of a normal pancreas. (B) Gross morphology of a decellularized pancreas. (C) HE staining shows the presence of blue nuclei in a normal pancreas. (D) HE staining shows the absence of blue nuclei in a decellularized pancreas. Please click here to view a larger version of this figure.
Discussion
The proposed protocol, using perfusion of SDC and Triton X-100 at 4 °C, will decellularize whole porcine pancreas successfully. The challenge in this technique is the dissection of the intact pancreas containing all the three lobes without damaging the parenchyma and its supplying vessels, as well as the ligation of the other vascular branches of the specimen in order to perfuse the organ without leakage. The porcine pancreas has a different anatomy compared to the human pancreas. It consists of three lobes and stays in close contact with the small intestine by partly surrounding it. We dissected the duodenum together with the pancreas, as several small blood vessels from pancreas connect the intestine. During the optimization studies, blunt cutting of these blood vessels has shown leakage and an incomplete perfusion of solutions.
Since the aorta connects to the pancreas through the coeliac and superior mesenteric arteries, we chose the aorta as an inlet to keep the perfusion simple by only using one cannula and, therefore, one inlet. In our experience, ligation of the aorta above the CT and below the SMA will decrease the time of dissection and reduces the risk of damaging any of the two vessels. In addition to a heparinized pig, we also noticed that a perfusion of cold heparin via the aorta immediately after the dissection helps in achieving perfusion of solutions throughout the organ. We speculate if this occurs by preventing the formation of blood clots in the blood vessels. The initial perfusion of a pancreas with ultrapure water after dissection will lyse red blood cells and remove the blood remnants in the organ, thereby preventing the formation of blood clots. This period can also be used to find any unligated small branches of veins and arteries, as blood flow can be easily noticed above the background.
We chose to keep the whole decellularization procedure at cold temperatures (4 °C), as this will hinder the action of exocrine enzymes that release from exocrine cells of the pancreas. The exocrine enzymes, when not inhibited, can cause a deleterious effect on cells and the ECM, as they can digest cell membranes and proteins12. As freezing and thawing can effectively burst the cells, we included a freeze/thaw step, initially even before the perfusion of detergents4,13. The initial wash after thawing will remove the remnants of cell bursts. The detergent treatment we used is a mix of SDC and Triton X-100 at unusually high concentrations and at a high perfusion speed. We chose this approach to achieve faster decellularization by removing the exocrine cells that damage the ECM. We speculate that a hard and fast protocol is beneficial for pancreas decellularization, as less time will be available for pancreatic enzymes to interact with the ECM, thus preserving good ECM components. To preserve the ECM components, we also added serine protease inhibitor (PMSF) to the detergent solutions, as that will inhibit the activation of enzymes released from exocrine cells14. Sodium azide is added to all decellularization solutions, as it acts as a bacteriostatic agent, thereby inhibiting the chance of bacterial contamination15.
The pancreas decellularized following this protocol showed a preservation of ECM structures and the ECM proteins collagen and elastin. However, a significant loss of glycosaminoglycans was noticed in the decellularized pancreas. The pancreas decellularized in this fashion also showed promise for the attachment of human fetal pancreatic stem cells and the expression of exocrine and endocrine markers in pieces recellularized for 14 days12. However, to generate an intact and functional pancreas, further research is required in evaluating correct cell sources, cell types, cell seeding strategies, and bioreactor culture.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was financed by a grant from the Swedish Government LUA ALF to S.S.H.
Materials
| 4mm DLP arteriotomy cannula | Medtronic | 31104 | |
| 2ml Unlabelled pipette | vWR | 612-3720 | |
| Degasser | Biotech AB | 0001-6484 | |
| DNase-I | Worthington | LS0020007 | |
| Dulbecco's PBS with CaCl2 and MgCl2 | Sigma Aldrich | D8662 | |
| EDTA disodium salt dihydrate | AlfaAesar | A15161.OB | |
| Heparin | Leo | 387107 | |
| Luer Male with 1/8" ID Barb | Oina | LM-2PP-QC | For 3X5mm silicon tube |
| Peristaltic pump | Oina | SP-1X4 | |
| PMSF | Roche | 10837091001 | Unstable in aqueous solution. Should be added fresh before perfusion. |
| Potassium chloride | Sigma Aldrich | P5405 | |
| Potassium hydrogen phosphate | Sigma Aldrich | P9791 | |
| SDC | Sigma Aldrich | 30970 | |
| Silicon tube 3X5mm | VWR | 2280706 | |
| Sodium Azide | Sigma Aldrich | 71290 | |
| Sodium chloride | Sigma Aldrich | 13423 | |
| Sodium hydrogen phosphate | Merck | 71640-M | |
| Suture | Vömel | 14817 | |
| Syrringe 50mL | Becton Dickinson | 300137 | |
| Triton-X-100 | AlfaAesar | A16046.OF |
References
- Yang, H. K., Yoon, K. H. Current status of encapsulated islet transplantation. Journal of Diabetes and its Complications. 29 (5), 737-743 (2015).
- Badylak, S. F., Taylor, D., Uygun, K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annual Review of Biomedical Engineering. 13, 27-53 (2011).
- Crapo, P. M., Gilbert, T. W., Badylak, S. F. An overview of tissue and whole organ decellularization processes. Biomaterials. 32 (12), 3233-3243 (2011).
- Hynes, R. O. The evolution of metazoan extracellular matrix. Journal of Cell Biology. 196 (6), 671-679 (2012).
- Scarritt, M. E., Pashos, N. C., Bunnell, B. A. A review of cellularization strategies for tissue engineering of whole organs. Frontiers in Bioengineering and Biotechnol. 3, 43 (2015).
- Goh, S. K., et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 34 (28), 6760-6772 (2013).
- Wu, D., et al. 3D Culture of MIN-6 Cells on Decellularized Pancreatic Scaffold. In Vitro and In Vivo Study. Biomed Research International. 2015, 432645 (2015).
- Peloso, A., et al. The Human Pancreas as a Source of Protolerogenic Extracellular Matrix Scaffold for a New-generation Bioartificial Endocrine Pancreas. Annals of Surgery. 264 (1), 169-179 (2016).
- Mirmalek-Sani, S. H., et al. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials. 34 (22), 5488-5495 (2013).
- Taylor, M. J., Baicu, S., Greene, E., Vazquez, A., Brassil, J. Islet isolation from juvenile porcine pancreas after 24-h hypothermic machine perfusion preservation. Cell Transplantation. 19 (5), 613-628 (2010).
- Elebring, E., Kuna, V. K., Kvarnstrom, N., Sumitran-Holgersson, S. Cold-perfusion decellularization of whole-organ porcine pancreas supports human fetal pancreatic cell attachment and expression of endocrine and exocrine markers. Journal of Tissue Engineering. 8, 2041731417738145 (2017).
- Gilpin, A., Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Research International. 2017, 9831534 (2017).
- James, G. T. Inactivation of the protease inhibitor phenylmethylsulfonyl fluoride in buffers. Analytical Biochemistry. 86 (2), 574-579 (1978).
- Lichstein, H. C., Soule, M. H. Studies of the Effect of Sodium Azide on Microbic Growth and Respiration: I. The Action of Sodium Azide on Microbic Growth. Journal of Bacteriology. 47 (3), 221-230 (1944).

