Human Blastocyst Biopsy and Vitrification
Summary
Blastocyst biopsy and vitrification are required to efficiently conduct preimplantation genetic testing. An approach entailing the sequential opening of the zona pellucida and retrieval of 7-8 trophectoderm cells in day 5-7 post-insemination limits both the number of manipulations required and the exposure of the embryo to sub-optimal environmental conditions.
Abstract
Blastocyst biopsy is performed to obtain a reliable genetic diagnosis during IVF cycles with preimplantation genetic testing. Then, the ideal workflow entails a safe and efficient vitrification protocol, due to the turnaround time of the diagnostic techniques and to transfer the selected embryo(s) on a physiological endometrium in a following natural cycle. A biopsy approach encompassing the sequential opening of the zona pellucida and retrieval of 5-10 trophectoderm cells (ideally 7-8) limits both the number of manipulations required and the exposure of the embryo to sub-optimal environmental conditions. After proper training, the technique was reproducible across different operators in terms of timing of biopsy (~8 min, ranging 3-22 min based on the number of embryos to biopsy per dish), conclusive diagnoses obtained (~97.5%) and live birth rates after vitrified-warmed euploid blastocyst transfer (>40%). The survival rate after biopsy, vitrification and warming was as high as 99.8%. The re-expansion rate at 1.5 h from warming was as high as 97%, largely dependent on the timing between biopsy and vitrification (ideally ≤30 min), blastocyst morphological quality and day of biopsy. In general, it is better to vitrify a collapsed blastocyst; therefore, in non-PGT cycles, laser-assisted artificial shrinkage might be performed to induce embryo collapse prior to cryopreservation. The most promising future perspective is the non-invasive analysis of the IVF culture media after blastocyst culture as a putative source of embryonic DNA. However, this potential avant-garde is still under investigation and a reliable protocol yet needs to be defined and validated.
Introduction
The main goal of modern human embryology is to maximize the number live births per stimulated cycle and reduce costs, time and efforts to achieve a pregnancy. To accomplish this goal, validated approaches for embryo selection should be employed to identify reproductively competent embryos within a cohort obtained during an IVF cycle. According to the latest evidences, blastocyst culture1 combined with comprehensive chromosomal testing and vitrified-warmed euploid embryo transfer (ET) is the most efficient framework to increase IVF efficiency2. Clearly, aneuploidy testing requires an embryonic specimen, which at present is mostly represented from few cells retrieved from the trophectoderm (TE), i.e., the section of the blastocyst that gives origin to embryo annexes (e.g., the placenta) during pregnancy. Beyond karyotype analysis, also single gene mutations might be assessed from a TE biopsy as part of a clinical strategy known as preimplantation genetic testing (PGT; -A for aneuploidies, -SR for structural chromosomal rearrangements, -M for monogenic diseases). Other oocyte/embryo biopsy methods have been theorized and adopted clinically across the last decades, namely polar bodies biopsy and blastomere biopsy. However, their use is reduced nowadays since their procedural drawbacks (e.g., higher workload and risk for reproductive impact) and diagnostic limitations (e.g., single cell analysis issues) implicitly hinder a sufficient balance between costs, risks and benefits (for a review see3).
In this paper, one of the main protocols for TE biopsy is thoroughly described together with the subsequent vitrification, warming and transfer procedures required. The workflow here outlined is ideal for a busy PGT unit.
As already described previously by our group4,5, the procedure involves the sequential opening of the zona pellucida of fully-expanded blastocysts and removal of few TE cells (on average 7-8). Compared to the day 3 laser-assisted hatching-based blastocyst biopsy method6, this procedure might ease the daily schedule of an IVF unit where delicate procedures, such as blastocyst biopsy and vitrification, must be timely performed. As soon as the blastocyst reaches its full expansion, the biopsy can be carried out by selecting the TE cells to remove, thereby preventing the risk of herniation of the inner cell mass (ICM), which would otherwise render the procedure challenging. In literature, a third protocol of blastocyst biopsy has been also described, which involves laser-assisted hatching being performed once the embryo has already reached the blastocyst stage, few hours before the procedure5,7. However, this approach is more time-consuming and mainly suits IVF units that are implementing TE biopsy in the hands of limitedly experienced operators and in view of a moderate-low daily workload.
Intracytoplasmatic sperm injection (ICSI)8 should be a consolidated technique if aiming at conducting genetic analyses in IVF. Similarly, a proper culture system to safely harvest embryos to the blastocyst stage is crucial for the implementation of TE biopsy strategy. An adequate number of incubators, as well as the use of low oxygen tension are key prerequisites to this end, not to compromise the blastocyst rate9. At the same time, an efficient cryopreservation program is needed to safely manage a PGT cycle. In the last decade, the implementation of vitrification has boosted embryo cryo-survival rates even up to >99%10,11. This provided sufficient time to perform genetic testing and postpone embryo transfer to the following menstrual cycle, on a non-stimulated and probably more receptive endometrium12.
Both TE biopsy and vitrification are demanding tasks requiring stringent skills and their effectiveness might vary across unexperienced operators. A specific training period is therefore advocated before allowing each operator to perform these procedures clinically; moreover, the maintenance of the operators’ skills should be assessed periodically by monitoring key performance indicators (KPI) for cryopreservation and biopsy procedures. Each IVF clinic should set internal KPIs to this end, which must approximate the ones published by international consortia and/or the outcomes published by reference laboratories.
TE biopsy, vitrification-warming and witnessing procedures are validated techniques at our unit, that have been standardized across all the operators involved as reported in three previous publications11,13,14.
Protocol
The protocol for human blastocyst biopsy, here described, follows the guidelines of G.EN.E.R.A. Human Research Ethic Committee.
NOTE: Refer to the Table of Materials for materials required. Further material required entails laboratory footwear and outfit, surgical facemask, hair cover, surgical gloves, a permanent non-toxic marker, forceps and disinfectant. The use of surgical gown, disposable surgical gloves, facemask, hair cover is mandatory to prevent risk of contamination. All the working areas, as well as the equipment involved in the process, must be cleaned thoroughly with laboratory disinfectant (e.g., Oosafe) before starting any procedure. All consumables and media used should be sterile and individually packaged or aliquoted. It is suggested to use a dedicated workstation for biopsy and tubing and limit the access of the area only to the operators involved in the procedure (embryologist and witness).
1. Preparation on the Day Before the Biopsy Procedure
- Prepare the post biopsy culture dish.
- Place 6 drops of 20 µL IVF culture medium (Table of Materials) in an IVF culture dish and overlay with 6 mL of prewarmed mineral oil for embryo culture (Table of Materials).
- Add another 10 µL of IVF culture medium to each drop. Incubate overnight at 37 °C in a controlled atmosphere (5% O2, 6% CO2).
- Prepare an IVF dish 4-well plate for rinsing the blastocyst after biopsy.
- Place 600 µL of IVF culture medium in the first two wells and overlay with 300 µL of pre-warmed mineral oil for embryo culture.
- Incubate overnight at 37 °C in a controlled atmosphere (5% O2, 6% CO2).
- Dispense 1.5 µL of loading solution (Table of Materials) in each PCR tube to be used for TE cells tubing, spin it and keep it at 4 °C.
2. Preparation on the Day of the Biopsy Procedure
- Prepare the biopsy dish.
- Place 3 drops of 10 µL HEPES-buffered medium (supplemented with human serum albumin) (Table of Materials) in a row in an IVF culture dish.
- Repeat step 1.1.1 according to the number of blastocysts available (up to 4 blastocysts per dish) and overlay with 6 mL of pre-warmed mineral oil for embryo culture.
- Incubate the dish at 37 °C for at least 1 h before performing the biopsy procedure.
3. Blastocyst Selection and Grading
- Grade the blastocyst according to its expansion and the morphological appearance of ICM and TE (Figure 1).
NOTE: The grading criteria have been adapted from Gardner and Schoolcraft15 and previously described by Capalbo et al. and Cimadomo et al.4,11.- Define the size and expansion grade as: A for a fully-hatched blastocyst, B for a hatching blastocyst, C for a fully expanded blastocyst, and D for a not expanded blastocyst (these embryos are biopsied only if they did not expand further up to day 7).
- Define ICM grade: 1 for noticeable ICM with several strictly-packed cells; 2 for discernable with several but roughly-packed cells; 3 for difficult to distinguish with very few low-quality cells.
- Define TE grade as follows: 1 for well-organized epithelium with several cells; 2 for loose epithelium with few cells; 3 for few and/or large low-quality cells.
4. Trophectoderm Biopsy
- Perform TE biopsy on all the viable fully-expanded blastocysts (preferably C grade for size and expansion).
- Set holding and biopsy pipettes for each biopsy procedure. The recommendations for the biopsy pipette are the following: 30 µm internal diameter, 35° bend angle, 0.75 mm distance tip to bend.
- Label the biopsy dish with patient’s details (woman’s name and surname, date of birth and ID) and then number each drop with embryo and cycle IDs. Use a permanent non-toxic marker.
- Transfer the blastocyst with a 300 µm stripping pipette to the first drop of the biopsy dish and rinse it in order to remove the excess of culture medium. Then move the blastocyst in the second drop of the biopsy dish.
- Move the dish to the inverted microscope and prime the biopsy pipette aspirating some medium from the third drop of the biopsy dish.
- At 20x magnification, orient the blastocyst to have a clear view of the ICM. When it is visualized at 7 o’clock (opposite to the area that will be targeted to remove the TE cells), secure the embryo on the holding pipette (Figure 2a).
- Focus on the zona pellucida and ascertain that both the pipettes and the blastocyst are on the same focal plane.
- Switch to the laser objective (pulse time 0.3 ms, 6.5 µm) and position the laser pointer on the zona pellucida at the opposite side of ICM. Drill the zona pellucida through 2−3 laser pulses (Figure 2b).
- Gently press the biopsy pipette against the zona pellucida and blow some medium through the breach to detach the TE cells from its internal surface and expedite blastocyst collapse (Figure 2c).
- Once the TE is detached (Figure 2d), enter through the hole (if required, make it wider with a last laser pulse) and aspirate few TE cells (ideally 7−813,16) into the biopsy pipette with gentle suction (Figure 2e).
- Slightly move the biopsy pipette backwards while applying a moderate suction to stretch the target cells (Figure 2f).
- Direct the laser towards the thinnest part of the aspirated cells and fire 2−5 laser pulses at the junctions between cells to separate the target cells from the body of the embryo (Figure 2g). The timing of laser pulses and the number of pulses can be adjusted according to the quality of the blastocyst; however, try to minimize them to avoid cell lysis.
- After the separation of the TE fragment from the blastocyst (Figure 2h), release it into the same biopsy drop far from the blastocyst. This is needed to prevent them from being sucked again into the biopsy pipette (Figure 2i).
- Release the blastocyst from the holding pipette and promptly raise both pipettes to prevent the fragment from sticking to them.
- Take a picture of the biopsied fragment for quality control purpose (Figure 3).
NOTE: If more than a single blastocyst is to be biopsied per procedure (up to four per biopsy dish), change the biopsy pipette with a new one to prevent cross-contamination between embryos. - Repeat steps 4.1−4.13.
- Move the biopsy dish back to the laminar flow hood.
- Label the post-biopsy culture dish with the couple ID, and each drop with the embryo and the cycle ID.
- In presence of a witness, rinse the blastocyst in clean IVF medium, and lastly move it to its corresponding drop of the post-biopsy dish.
- Move the post-biopsy dish to the incubator in a controlled atmosphere (37 °C, 6% CO2, 5% O2) until vitrification. It is advisable to perform vitrification within 30 min from the biopsy procedure, to prevent blastocyst re-expansion.
5. Tubing
NOTE: The whole procedure must be carried out in the presence of a witness and inside the laminar flow hood at room temperature. During the procedure, keep the PCR tubes in a cold tube rack on ice (Supplementary Figure 1).
- Label the PCR tubes with a permanent non-toxic marker.
NOTE: Labeling should be performed as requested by the genetic laboratory. Generally, patient’s name and surname (initials), couple ID, embryo and cycle ID (for instance: JD 12345 1.2 for the embryo N.1 of 2nd cycle belonging to Jane Doe whose couple ID is 12345). Embryo ID should be reported also in letters on the body of the tube. - Label the lid of a 60 mm x 15 mm culture dish (tubing dish) with the biopsied embryos’ IDs (Supplementary Figure 1) and prepare two 10 µL drops of biopsy washing solution (Table of Materials) in it.
- Prime the 140 µm stripping pipette (Supplementary Figure 1) with some biopsy washing solution from the second drop of the tubing dish.
- Place the biopsy dish under the stereomicroscope to easily visualize the TE fragment(s).
- Gently release some biopsy washing solution upon the TE fragment; then, load it into the stripping pipette.
- Move the TE fragment to the second drop of biopsy washing solution in the tubing dish and carefully rinse it 2−3 times.
- Transfer the TE fragment to the bottom of the PCR tube (previously labeled after it) with loading solution, paying attention to avoid touching its walls with the tip of the stripping pipette.
- Repeat the procedure from step 5.3 to step 5.7 for each TE fragment, paying attention to use a new capillary for every TE fragment.
- At the end of the tubing procedure, put all the PCR tubes in a mini centrifuge, and spin them for few seconds.
- Store the samples at -20 °C until shipping them to the referring genetic laboratory for testing.
6. Blastocyst Vitrification
- Vitrify collapsed blastocysts within 30 min from TE biopsy to prevent their re-expansion.
- Label the vitrification plate with woman’s details and the IDs of the blastocysts that must be vitrified.
- Label the vitrification support(s) with woman’s name and surname, couple ID, ID of the embryo that will be loaded on it, as well as date of the procedure. Special cryolabels are used which preserve their integrity even at very low temperature (-196 °C).
- At room temperature, dispense 0.3 mL of equilibration solution (ES) (Table of Materials) for each blastocyst that will be vitrified.
- In the presence of a witness, move the blastocyst in ES using the 300 µm stripping pipette.
- Leave the blastocyst in the ES for 13−15 min. After an initial shrinkage of the volume, a gradual re-expansion will be observed.
- Fill a small cooling rack with liquid nitrogen (LN2) and place it under the laminar flow hood.
- Dispense 300 μL of vitrification solution (VS) (Table of Materials) in the second well. After the blastocyst complete re-expansion, transfer it in the VS solution for 1 min and rinse it to dilute the ES.
- In presence of a witness, load the blastocyst on the vitrification support and take care of removing the excess of VS. A subtle film of solution should surround the blastocyst.
- Plunge the vitrification support into LN2 and move it energetically in order to reduce the risk of bubble formation close to the specimen.
- Place the protective cap while keeping the vitrification support submerged in the LN2.
- In the presence of a witness, move the vitrification support in a long term LN2 storage tank.
7. Artificial shrinkage of Non-biopsied Blastocysts
- If no TE biopsy is conducted, artificially collapse the blastocysts immediately before vitrification (Figure 4).
- Move the culture dish from the incubator to the inverted microscope and focus on the selected embryo.
- Switch to the laser objective (pre-set pulse: 0.3 ms, 6.5 μm), target the zona pellucida at the opposite side of ICM, then direct 1–2 laser pulses to the junctions between TE cells at a safe distance from the ICM. The blastocysts should collapse in less than 5 min.
- Proceed with the vitrification of the collapsed blastocyst as described in section 6.
8. Transferable Blastocyst Warming
- On the day of the ET, prepare the ET 4-well plate by placing 600 µL of pre-equilibrated IVF culture medium for each well. Incubate at 37 °C in a controlled atmosphere (5% O2, 6% CO2), while performing blastocyst warming.
- Label the warming dish with woman’s name and surname, couple ID, ID of the embryo that will be warmed in it.
- Dispense 1 mL of the thawing solution (TS) (Table of Materials) in an IVF one-well dish (Supplementary Figure 1) and warm it to 37 °C in a thermostat for at least 1 h before starting the procedure.
- Fill a small cooling support with LN2 and place it in the laminar flow hood in close proximity of the working area.
- Before starting the procedure, check the blastocyst information reported on the vitrification support. All the IDs should match the genetic report and the blastocyst to warm must be chosen among the transferable ones. A witness is required during the procedure.
- Take the one-well dish containing warmed TS out of the thermostat and place it on a heated stage under the stereomicroscope.
- Pull over the protective cap within the LN2 using forceps.
- Plunge quickly the tip of the vitrification support into the 37 °C TS.
- Under microscopic observation, gently move the vitrification support until the blastocyst is released from the tip.
- Leave the blastocyst for a total of 1 min in the TS, paying attention to keep it at the bottom of the TS.
- At room temperature dispense 200 μL of dilution solution (DS) (Table of Materials) on the first well of the vitrification plate.
- Transfer the blastocyst to DS and place it at the bottom of the well while releasing some TS on the top of it to create a sort of gradient. Leave it for 3 min.
- Dispense 200 μL of washing solution (WS) in 2 different wells (WS1 and WS2).
- Transfer the blastocyst to WS1 and place it on the bottom of the well while releasing some DS medium on the top of it. Then transfer the blastocyst to WS2 and leave it undisturbed for 1 min.
- Label the post-warming ET plate with woman’s name and surname, couple ID, ID of the embryo that will be cultured in it.
- In the presence of a witness, transfer the warmed blastocyst to pre-equilibrated culture medium in the post-warming ET plate.
- Rinse the blastocyst in the first well of the ET plate and place it in the second well.
- Transfer the post-warming culture dish to the incubator in a controlled atmosphere (37 °C, 6% CO2, 5% O2) and culture the blastocyst for at least 1.5 h before checking its survival and re-expansion.
NOTE: Figure 5 shows examples of degenerated, cryo-survived but not re-expanded and cryo-survived and fully-expanded blastocysts.
9. EmbryoTransfer
- To perform ET, place the dish on the heated stage of the laminar flow hood, so the embryo is visible under the microscope at a low magnification.
- Take about 0.4 mL of IVF culture medium. Secure the syringe tip firmly into the receiving end of the internal catheter and then release the medium up to 0.1 mL.
- Aspirate culture medium until observing the embryos entering the catheter (minimal amount of media: 10−15 μL).
- Place the catheter into the plastic case and go to the operating room and place the internal catheter into the external guide.
- Once reached the uterus, push the syringe plunger to release the embryo(s). Release very slowly the medium until the plunger is reading 0.1 mL.
- Take the catheter back to the lab and check under the microscope that the embryo has been transferred.
Representative Results
Figure 6 represents a scheme of all the outcomes of a biopsy procedure that can be adopted to standardize the protocol and monitor the performance of each operator. The main procedural outcome is the timing to complete the biopsy/biopsies; the main technical outcome is the quality of the plot produced after genetic testing that might result in either a conclusive or inconclusive diagnosis, the latter of which requires a re-biopsy of the undiagnosed blastocyst; the main biological outcome is the rate of cryo-survival and re-expansion versus degeneration after warming; lastly, the main clinical outcome is the live birth rate after vitrified-warmed blastocyst transfer. In three previous studies we reported the KPIs defined at the center(s) for the technical, biological and clinical outcomes11,13,16. Hereafter, we instead report how the KPI for the timing of biopsy was defined. Moreover, the putative influence of the timing between biopsy and vitrification on the post-warming behavior of euploid blastocysts was also investigated.
In a 2 year period, a total of 1,544 trophectoderm biopsy procedures were conducted by 7 operators (Table 1). All biopsied blastocysts were then moved back to the incubator into a post-biopsy culture dish until vitrification. All the relevant data were collected in a relational database. All the timings of biopsy and between biopsy and vitrification were retrospectively obtained from the software of the IVF electronic witnessing system. The data were then exported and analyzed for statistics.
The cryo-survival rate of euploid blastocysts after trophectoderm biopsy and vitrification-warming was N = 571/572, 99.8%. The re-expansion rate at 1.5 h after warming was N = 556/571, 97.4%. Among the 15 not re-expanded blastocysts, one resulted in a live birth after being transferred in utero. The live birth rate after vitrified-warmed euploid single blastocyst transfer was N=227/572, 39.7%.
Definition of the ideal timing of biopsy
Table 1 summarizes the relevant data of the biopsy procedures conducted. Overall, 1.89 ± 1.03 (range 1-4) blastocysts were biopsied per procedure in 8.24 ± 4.23 min (range 3-22). The mean timing of biopsy varied because of both the number of blastocysts biopsied per procedure, from a minimum of 5.78 ± 2.94 min (range 3-16) when only one embryo was laid in the dish to a maximum of 12.93 ± 4.43 min (range 6-22) when the embryos sequentially biopsied were 4. Another relevant parameter was the operator involved in the procedure: the most expert (N = 443 procedures) was the fastest (7.41 ± 3.6 min, range 3-22), while the least experienced (N = 42) was the slowest (14.19 ± 4.24 min, range 6-22). Indeed, a generalized linear model entailing both the “number of blastocysts biopsied per procedure” and the “operator” variables perfectly explains the “timing of biopsy” with a R2 = 0.48 and a power = 1. This analysis was useful to define that ideally ~6 min is enough for a blastocyst biopsy procedure when only an embryo is laid in the dish, while ~9 min, ~12 min and ~13 min for 2, 3 and 4 blastocysts, respectively. Clearly, the whole procedure entails also moving the embryos from the culture to the biopsy dish and from the latter to the post-biopsy culture dish after the procedure, as well as changing the biopsy pipette between sequential biopsies.
Figure 7 plots the mean timing of biopsy for each operator along the study trimesters (from the 1st to the 8th). The dotted red line identifies the mean overall value of 8.24 min. Such a graph is useful to monitor the mean performance of each practitioner. For instance, the most expert operators (1 and 2) showed a constant decrease of this timing, which suggests a trend typical of a learning curve. All the operators from 3 to 6 were instead sufficiently constant in their performance around the mean overall value across the trimesters. Anytime they showed a peak in the mean value from a given trimester (e.g., operator 3 in the 7th trimester, operator 4 in 6th trimester, operator 5 in the 3rd trimester), they were warned in order to revise their performance. Operator 7 (i.e., the least experienced) showed timings typical of an embryologist that has just finished his/her training. Possibly, he/she will meet the standards internal to the lab as the expertise would increase.
Importantly, the time of biopsy was similar across re-expanded and not re-expanded euploid blastocysts at 1.5 h from warming (9.52 ± 4.23 min, range 3-22 versus 10.5 ± 5.68 min, range 4-22; t-test = 0.37). Likely, implanted (N = 229) and not implanted (N = 343) vitrified-warmed euploid blastocysts also showed comparable biopsy timings (9.77 ± 4.15 min, range 3-22 versus 9.41 ± 4.36 min, range 3-22; t-test = 0.39). Possibly then, a timing ≤22 min to biopsy up to 4 blastocysts does not affect embryo behavior after warming. Therefore, we defined this value as maximum threshold.
Similarly, no difference was shown in terms of live birth rate across the different biopsy operators, as already reported previously13 (Supplementary Table 1).
Another important parameter to monitor each operator’s performance is the rate of inconclusive results after diagnosis, which should be as close as possible to the general performance of each laboratory. Ideally this rate should not exceed 2.5% and might decrease with time due to an increasing expertise in biopsy and tubing procedures16. The target number of TE cells to retrieve are 7-8 according to two previous studies13,16. To this end, it is suggested to take a picture of the biopsied fragment for quality control purpose (see some examples in Figure 3). Such picture might be checked in case of inconclusive diagnoses to evaluate whether the cause was imputable to the dimension/quality of the fragment (i.e., low quality of the molecular analysis), to the tubing (i.e., DNA amplification failure) or to some issues in the processing of the sample in the genetic laboratory.
Definition of the ideal timing between biopsy and vitrification
In the study period, 572 euploid blastocysts were warmed to undergo an embryo transfer after a diagnosis of euploidy. Figure 8A shows each warmed blastocyst as a black circle distributed across the increasing timing between biopsy and vitrification and clustered in two groups according to the outcome under investigation: re-expanded or not re-expanded within 1.5 h from warming. All the blastocysts (N = 117/117) vitrified within 30 min, 97.6% (N = 245/251) of the blastocysts vitrified between 31-90 min, and 95.1% (N = 194/204) of the blastocysts vitrified beyond 90 min re-expanded, respectively (no re-expansion rates: 0%, 2.4% and 4.9%). Therefore, we set 30 min and 90 min as the early and late thresholds of time between biopsy and vitrification.
Figure 8B shows the re-expansion rate in the three groups (≤30 min, 31-90 min, >90 min) further sub-clustered according to the blastocyst quality and day of preimplantation development. Especially for poor quality and/or day 7 blastocysts, the timing between biopsy and vitrification seems crucial to achieve re-expansion after warming. Specifically, the odds-ratio of re-expansion after warming corrected for both blastocyst quality and day of biopsy in blastocysts vitrified within 30 min from biopsy versus blastocysts vitrified beyond 90 min was 3.05 (95% CI 1.01-9.4, p=0.05). Instead, the period in between these two thresholds (31-90 min) represented a grey area that might or might not have an impact.
Only 1 out of 15 not re-expanded blastocysts resulted in a live birth after transfer. Therefore, we lastly investigated the live birth rate achieved after warmed euploid single blastocyst transfer clustered in the three groups according to the timing between biopsy and vitrification. The highest live birth rate was achieved by transferring euploid blastocysts vitrified ≤30 min from the trophectoderm biopsy (N = 56/117, 47.9%). However, this result did not reach statistical significance when compared to the same outcome obtained either with blastocysts vitrified between 31 and 90 min (N = 92/251, 36.7%; Fisher’s exact test = 0.06), or with blastocysts vitrified >90 min from the biopsy (N=81/204, 39.7%; Fisher’s exact test = 0.16). Therefore, either a negative effect on blastocyst reproductive competence is negligible or the sample size in this dataset (N = 572) was insufficient to reach statistical significance.
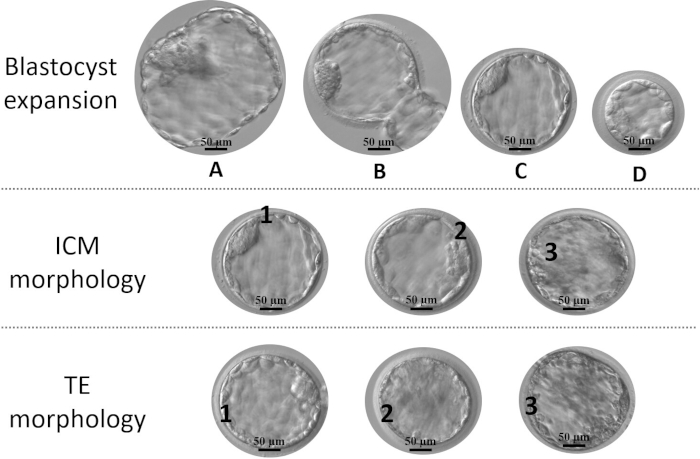
Figure 1: Parameters for blastocyst grading. Expansion: (A) fully hatched, (B) in hatching, (C) fully expanded, and (D) not expanded. The ideal stage is C, while a blastocyst D should be given more time to achieve full expansion, unless this stage is reached in day 7; Inner cell mass (ICM) morphological quality: 1 (noticeable with several strictly packed cells), 2 (discernable with several but roughly packed cells) and 3 (difficult to distinguish with very few low-quality cells); trophectoderm (TE) morphological quality: 1 (well-organized epithelium with several cells), 2 (loose epithelium with few cells) and 3 (few and/or large low-quality cells). Please click here to view a larger version of this figure.
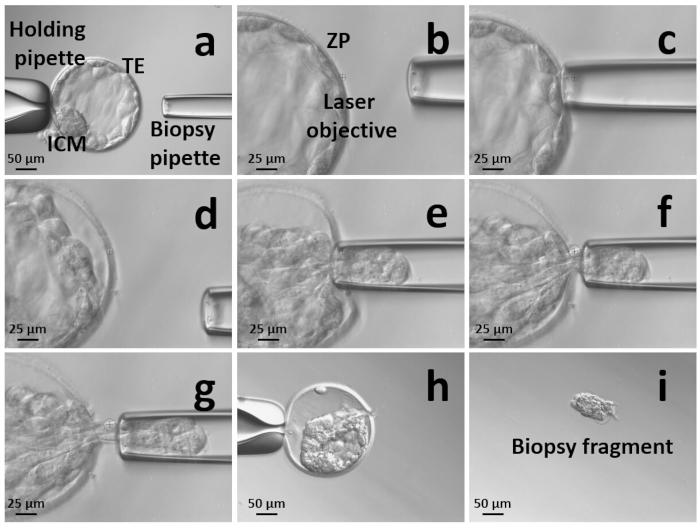
Figure 2: Summary of the sequential zona pellucida (ZP) opening and trophectoderm (TE) cells retrieval approach for blastocyst biopsy. (a) Orient the blastocyst with the inner cell mass (ICM) close to the holding pipette and far from the spot where the selected TE cells will be retrieved. Secure the blastocyst on the holding pipette; (b) open the ZP through 2-3 laser shots; (c) blow some culture media through the hole; (d) the blastocyst will detach from the ZP; (e) enter the ZP and suck 5-10 TE cells in the biopsy pipette; (f) move backwards with the biopsy pipette to stretch the selected fragment and expose the junctions between the cells; (g) fire at the junctions between the cells and continue stretching the fragment until the TE cells are released from the body of the blastocyst; (h) the blastocyst after TE biopsy is collapsed; (i) take a picture of the biopsy fragment for quality control and transfer it to the PCR tube that will be sent to the genetic laboratory. Please click here to view a larger version of this figure.
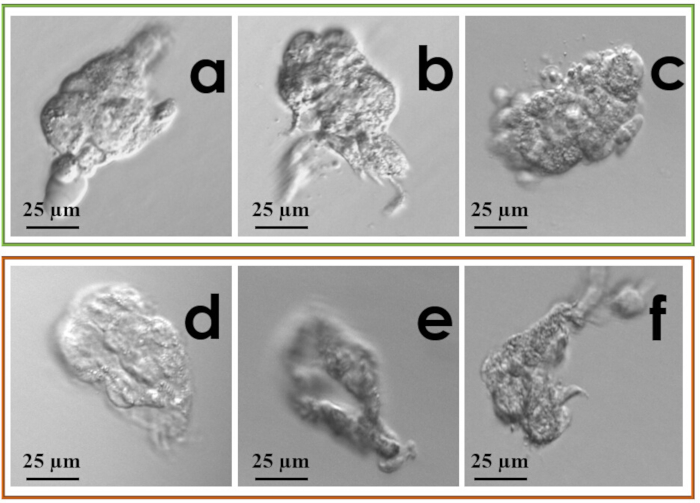
Figure 3: Examples of biopsy fragments: (a-c) desirable fragments; (d) lysed fragment; (e) small fragment with degenerated cells; (f) small, partially lysed and degenerated fragment. Please click here to view a larger version of this figure.
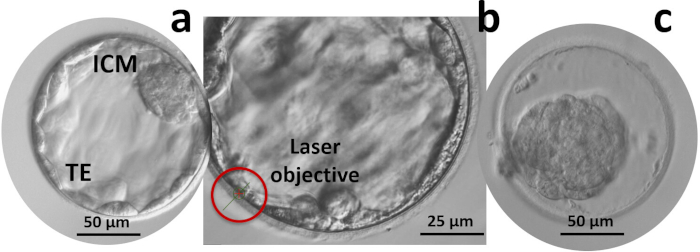
Figure 4: Artificial shrinkage. (a) Orientate the blastocyst so that the inner cell mass (ICM) is far from the targeted section of the trophectoderm (TE); (b) Fire 2-3 laser shots in a row at the junctions between TE cells and moving outwards; (c) Wait for the blastocyst to collapse before starting vitrification. Please click here to view a larger version of this figure.
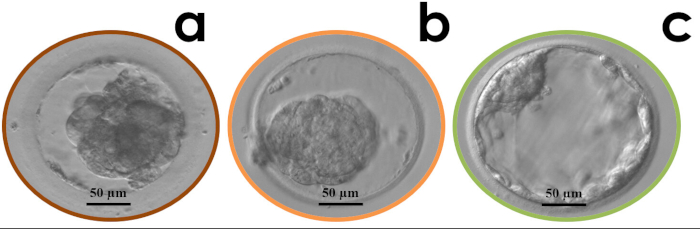
Figure 5: Examples of blastocyst degeneration (a), cryo-survival but no re-expansion (b) and cryo-survival and full re-expansion (c) 1.5 h post-warming. Please click here to view a larger version of this figure.
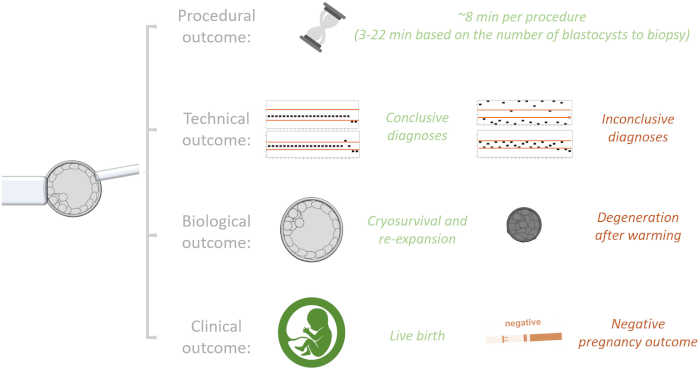
Figure 6: Summary of the different outcomes of trophectoderm biopsy that might be used to monitor the performance of an operator and define the key performance indicators internal to each laboratory. The main procedural outcome is the timing of biopsy. The main technical outcome is the rate of conclusive (euploid or aneuploid) and inconclusive diagnoses (re-biopsy required) obtained; the latter might be caused by DNA amplification or low-quality molecular data, both resulting in not-interpretable chromosome copy number profile plots. The main biological outcome is the rate of cryo-survival and re-expansion or degeneration after biopsy, vitrification and warming. The main clinical outcome is the rate of live births or negative pregnancy outcomes achieved after vitrified-warmed blastocyst transfer. Of note, while the procedural outcome is exclusively dependent on the operator and the number of blastocysts to biopsy per procedure, all other outcomes might be affected from other confounders independent from the biopsy operator (e.g., the steps and operators involved in the molecular analysis, the morphological quality of the blastocyst, the day of biopsy) that should be accounted to properly evaluate his/her performance. Please click here to view a larger version of this figure.
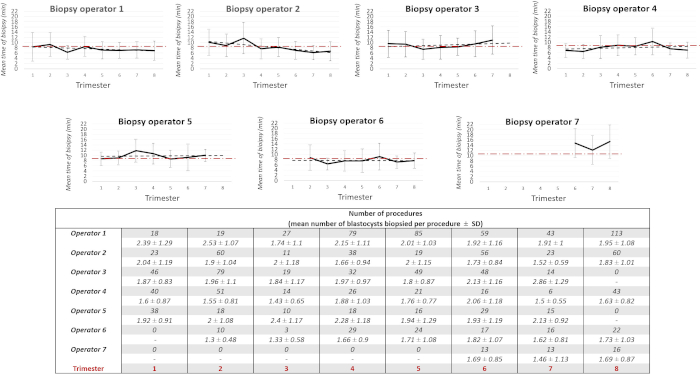
Figure 7: Mean timing of biopsy per operator across the 8 study trimesters. The table summarizes the related number of procedures and the mean number of blastocysts biopsied per procedure by each operator in the 8 study trimesters. The dotted red line within each graph represents the overall mean timing of biopsy (8.24 min). The error bars are the standard deviations. Please click here to view a larger version of this figure.
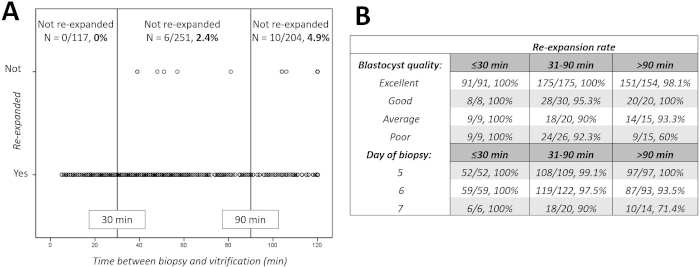
Figure 8: Re-expansion after warming versus timing between biopsy and vitrification. (A) shows not re-expanded and re-expanded blastocysts 1.5 hr after warming. Each blastocyst is represented by a black circle across the increasing timings. The vertical continuous black lines represent 30 min set as early threshold and 90 min set as late threshold. (B) shows the re-expansion rates in the three groups (timing between biopsy and vitrification: ≤30 min, 31-90 min, >90 min) further clustered according to blastocyst quality and day of biopsy. Please click here to view a larger version of this figure.
| N of procedures | N blastocysts biopsied per procedure | Mean timing of biopsy ± SD, range (min) | |
| Operator 1 | 443 | 2.01 ± 1.09, 1-4 | 7.41 ± 3.6, 3-22 |
| 195 | 1 | 4.75 ± 1.96, 3-16 | |
| 111 | 2 | 7.83 ± 2.45, 3-18 | |
| 71 | 3 | 10.27 ± 2.41, 4-16 | |
| 66 | 4 | 11.48 ± 3.81, 6-22 | |
| Operator 2 | 290 | 1.81 ± 0.98, 1-4 | 7.87 ± 4.13, 3-22 |
| 142 | 1 | 5.69 ± 3.32, 3-16 | |
| 89 | 2 | 8.48 ± 2.79, 3-18 | |
| 30 | 3 | 11.37 ± 3.72, 4-20 | |
| 29 | 4 | 13.1 ± 3.89, 9-22 | |
| Operator 3 | 287 | 1.98 ± 1.05, 1-4 | 9.10 ± 4.65, 3-22 |
| 121 | 1 | 6 ± 2.19, 3-15 | |
| 89 | 2 | 9.6 ± 3.87, 3-22 | |
| 38 | 3 | 12.66 ± 4.55, 4-22 | |
| 39 | 4 | 14.13 ± 4.8, 6-22 | |
| Operator 4 | 217 | 1.66 ± 0.87, 1-4 | 7.58 ± 3.45, 3-22 |
| 118 | 1 | 5.58 ± 1.96, 3-14 | |
| 66 | 2 | 8.92 ± 2.91, 4-22 | |
| 21 | 3 | 11.48 ± 2.34, 5-16 | |
| 12 | 4 | 13 ± 4.26, 6-19 | |
| Operator 5 | 144 | 2.03 ± 1.08, 1-4 | 9.43 ± 4.24, 3-22 |
| 59 | 1 | 6.15 ± 2.5, 3-16 | |
| 43 | 2 | 10.07 ± 2.73, 6-16 | |
| 20 | 3 | 12.6 ± 2.89, 9-18 | |
| 22 | 4 | 14.09 ± 4.43, 6-22 | |
| Operator 6 | 121 | 1.67 ± 0.94, 1-4 | 7.79 ± 3.93, 3-22 |
| 70 | 1 | 6.19 ± 2.95, 3-16 | |
| 32 | 2 | 8.12 ± 1.72, 3-11 | |
| 9 | 3 | 12.78 ± 3.31, 9-18 | |
| 10 | 4 | 13.5 ± 6.19, 6-22 | |
| Operator 7 | 42 | 1.62 ± 0.94, 1-4 | 14.19 ± 4.24, 6-22 |
| 27 | 1 | 11.85 ± 5.53, 6-16 | |
| 6 | 2 | 16.5 ± 3.73, 11-22 | |
| 7 | 3 | 19.86 ± 3.34, 13-22 | |
| 2 | 4 | 19 ± 4.24, 16-22 | |
| Total | 1544 | 1.89 ± 1.03, 1-4 | 8.24 ± 4.23, 3-22 |
| 732 | 1 | 5.78 ± 2.94, 3-16 | |
| 436 | 2 | 8.85 ± 3.14, 3-22 | |
| 196 | 3 | 11.72 ± 3.70, 4-22 | |
| 180 | 4 | 12.93 ± 4.43, 6-22 |
Table 1: Total mean timing of biopsy and mean number of blastocysts biopsied in each procedure according to biopsy operator. The mean timing of biopsy has been also shown according to each sequential number of blastocysts biopsied per procedure. A generalized linear model that includes both the “biopsy operator” and “number of blastocysts biopsied per procedure” variables perfectly correlates with the “timing of biopsy” (R2 = 0.48, power = 1).
Supplementary Figure 1: Main devices and supports required for the procedure. Please click here to download this file.
Supplementary Table 1: Logistic regression analysis does not show any significant association between the biopsy operator and live birth after vitrified-warmed euploid blastocyst transfer. Please click here to download this file.
Discussion
Only well-experienced skilled embryologists who have completed their training period should perform both TE biopsy and blastocyst vitrification. Furthermore, a witness is required to monitor the procedures and guarantee an efficient traceability during i) the movements of the biopsied blastocyst from the biopsy dish (Supplementary Figure 1) to the post-biopsy dish (Supplementary Figure 1), then to the vitrification plate (Supplementary Figure 1) and lastly to the vitrification support (Supplementary Figure 1); ii) the transfer of biopsied TE cells from the biopsy dish to the PCR tube (Supplementary Figure 1); iii) the warming and transfer steps post-diagnosis. For a detailed description of all witnessing steps refer to a failure modes and effects analysis (FMEA) previously published14.
All methods described in this paper respect the local regulation (Italian Law 40/2004). According to the Law, in fact, the couple can request to be informed about the health status of the embryos they produced during the IVF cycle. In this regard, a detailed informed consent for PGT must be signed from both partners.
In this paper we described how to implement blastocyst biopsy for PGT in a busy laboratory routine. The application of blastocyst biopsy approach has been an important advancement in the last decade in IVF. First reported by de Boer and colleagues in 200417, it has been soon recognized as a more effective and informative procedure compared to cleavage stage and polar body biopsy approaches3. The value of this procedure mainly resides in a reduction of the technical burdens, but also in a lower incidence of chromosomal mosaicism at this stage of development18,19. Moreover, the removal of few TE cells from a blastocyst has been suggested as a safer procedure than the removal of one blastomere from a cleavage stage embryo. Indeed, in a randomized non-selection study the former approach did not result in any impact on embryo implantation potential, while the latter involved a significant ~20% reduction20.
The protocol mostly used worldwide entails laser-assisted zona opening in day 3 post-insemination. No randomized controlled trial has been conducted to date to compare the different blastocyst biopsy approaches. However, it is reasonable that the lower the number of manipulations and exposures of the growing embryos to suboptimal environmental conditions, the lower the potential invasiveness of the protocol. Moreover, the presence of a hole in the zona pellucida from day 3 of development might affect blastocyst expansion and cause the herniation of ICM cells together with TE cells. For these reasons, we set and implemented the protocol described here that entails the sequential laser-assisted zona breaching and TE cells retrieval as soon as the blastocyst reaches full expansion. Also a different protocol exists that entails a day 5 or 6 assisted hatching5,7. Specifically, the drilling of the zona is performed on day 5 or 6 of preimplantation development on the opposite side with respect to the ICM; the blastocyst is then moved back to the incubator waiting for the spontaneous herniation of TE cells. Clearly, periodic monitoring of the blastocyst must be provided in the following hours, to biopsy it as soon as the TE will start herniating, as well as to prevent the embryo from hatching completely. If on the one hand unskilled practitioners can easily implement this alternative biopsy strategy, on the other hand it is not suitable for a busy laboratory performing several procedures per day. The sequential zona opening and blastocyst biopsy protocol described here is instead less time-consuming and allows a shorter hands-on time and a higher flexibility to schedule the daily activity in the laboratory so to coordinate the timeframes dedicated to biopsy and to vitrification procedures.
Poor quality blastocysts might be more complex to biopsy since the TE cells can be sticky, but more laser shots coordinated with the stretching of the fragment to expose the junctions between the cells are sufficient to remove it from the body of the blastocyst. Fully hatched blastocyst can be biopsied like blastocysts enclosed in the zona pellucida, but they might be trickier to vitrify and warm. In case of inconclusive diagnoses after biopsy, the data reported to date are concordant that no harm seems to derive from a re-biopsy and a following vitrification-warming cycle16,21.
Vitrification is currently used in the center to perform blastocyst cryopreservation since it has been consistently reported safer, more efficient and less time-consuming than slow freezing protocol from a review and meta-analysis of the most recent literature10.
Once the procedure was well-established and the operators properly trained, we performed a retrospective analysis to define the ideal procedural timings for both blastocyst biopsy and vitrification procedures (summarized here in the representative results). As final outcomes, we have assessed the re-expansion rate of euploid blastocyst evaluated at 1.5 h after warming and the live birth rate achieved after vitrified-warmed euploid single embryo transfer. No case of blastocyst degeneration was observed after biopsy, and just 0.2% (N = 1/572) of degeneration rate was reported after warming, confirming the reliability of the biopsy and vitrification approaches adopted. According to the analysis, the timing required to perform blastocyst biopsy does not affect either euploid blastocysts’ viability after warming defined as re-expansion rate, or reproductive potential defined as live birth rate. Although various timings can be observed across operators with different expertise, we may consider blastocyst biopsy safe when the procedure is completed in about 8 min, in a range from 3 to 22 min depending on the number of embryos per procedure (however no data are available for biopsy procedures performed in longer intervals). The mean time spent for blastocyst biopsy from each single operator should be periodically (at least every three months) monitored as KPI. Alongside, the rate of inconclusive diagnosis and the live birth rate after vitrified-warmed euploid blastocyst transfer should be also addressed. Lastly, we outlined the ideal timing between biopsy and vitrification. Since we observed the highest re-expansion rate after warming when blastocysts were vitrified within 30 min from the biopsy, we suggest this value as the ideal threshold. Specifically, the longer the time between biopsy and vitrification, the more the biopsied blastocyst will re-expand before being cryopreserved. This might be harmful for cryo-survival, especially when dealing with poor-quality and/or day 7 blastocysts11. Nevertheless, no significant impact on the clinical outcomes was observed even if vitrification was delayed beyond 90 min. Therefore, in sporadic occasions, such timings might be allowed.
The method chosen to retrieve a specimen for PGT should not affect embryo viability, should involve reliable and informative results, should be clinically effective and should be easy to implement thereby reducing the costs and the laboratory workload. Blastocyst biopsy fulfills all these prerequisites. Nonetheless, it still is an invasive procedure that must be performed by skilled operators in a well-equipped laboratory. Currently, the avant-garde of a non-invasive PGT (niPGT) approach is under investigation. Perhaps, in the future, the spent culture media after IVF might be analyzed to conduct chromosomal and/or genetic testing. This is an intriguing future perspective since the costs for the IVF clinic would be lower and all the workload entailed by embryo biopsy would be sidestepped. However, the reliability and reproducibility of spent media analysis for genetic testing are still to be assessed22,23,24, therefore more efforts must be invested to define and validate a protocol that could suit all IVF clinics performing PGT worldwide.
Disclosures
The authors have nothing to disclose.
Acknowledgements
AG and RM collected the data and drafted the manuscript. DC analyzed the data, drafted the representative results, performed the statistics and revised the manuscript. FMU and LR provided critical discussion of the results and of the whole manuscript.
Materials
| Equipment | |||
| Cold tube rack | Biocision | XTPCR96 | |
| Electronic pipette controller | Fisher Scientific | 710931 | |
| Flexipet adjustable handle set | Cook | G18674 | Stripper holder |
| Gilson Pipetman | Gilson | 66003 | p20 |
| IVF Electronic Witness System | CooperSurgical Fertility & Genomic Solutions | RI Witness ART Management System | |
| Inverted microscope | Nikon | Eclipse TE2000-U | |
| Laminar Flow Hood | IVF TECH | Grade A air flow | |
| Laser objective | RI | Saturn 5 | |
| Microinjectors | Nikon Narishige | NT-88-V3 | |
| Mini centrifuge for PCR tubes | Eppendorf | CSLQSPIN | for 0.2ml PCR tubes |
| Stereomicroscope | Leica | Leica M80 | |
| Thermostat | Panasonic | MCO-5AC-PE | |
| Tri-gas incubator | Panasonic | MCO-5M-PE | 02/CO2 |
| Consumables | |||
| Biopsy pipette | RI | 7-71-30FB35720 | 30µm ID, flat 35°C |
| Cryolock | Cryolock | CL-R-CT | |
| CSCM complete | Irvine Scientific | 90165 | IVF culture medium supplemented with HSA |
| Embryo Transfer Catheter | Cook | G17934 | |
| Flexipet pipette | Cook | G26712 | 140µm stripping pipette tip |
| Flexipet pipette | Cook | G46020 | 300µm stripping pipette tips |
| Holding pipette | RI | 7-71-IH35/20 | 30µm ID, flat 35°C |
| Human Serum Albumin | Irvine Scientific | 9988 | |
| IVF One well dish | Falcon | 353653 | |
| Mineral Oil for embryo culture | Irvine Scientific | 9305 | |
| Modified HTF Medium | Irvine Scientific | 90126 | Hepes-Buffered medium |
| Nuclon Delta Surface | Thermofisher scientific | 176740 | IVF dish 4-well plate with sliding lid |
| Primaria Cell culture dish | Corning | 353802 | 60x15mm |
| Reproplate | Kitazato | 83016 | |
| Serological pipette | Falcon | 357551 | 10ml |
| Sterile disposable Gilson tips | Eppendorf | 0030 075.021 | 200µl |
| Tubing Kit | Provided by the genetic lab | PCR tubes (0.2mL), loading solution, biopsy washing solution | |
| Vitrification media | Kitazato | VT801 | Equilibration and vitrification solutions |
| Warming media | Kitazato | VT802 | Thawing and dilution solutions |
References
- Glujovsky, D., Farquhar, C., Quinteiro Retamar, A. M., Alvarez Sedo, C. R., Blake, D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database of Systematic Reviews. (6), CD002118 (2016).
- Dahdouh, E. M., Balayla, J., Garcia-Velasco, J. A. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertility and Sterility. 104 (6), 1503-1512 (2015).
- Cimadomo, D., et al. The Impact of Biopsy on Human Embryo Developmental Potential during Preimplantation Genetic Diagnosis. Biomedical Research International. 2016, 7193075 (2016).
- Capalbo, A., et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Human Reproduction. 29 (6), 1173-1181 (2014).
- Capalbo, A., et al. Implementing PGD/PGD-A in IVF clinics: considerations for the best laboratory approach and management. Journal of Assisted Reproduction and Genetics. , (2016).
- McArthur, S. J., Leigh, D., Marshall, J. T., de Boer, K. A., Jansen, R. P. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertility and Sterility. 84 (6), 1628-1636 (2005).
- Kokkali, G., et al. Birth of a healthy infant following trophectoderm biopsy from blastocysts for PGD of beta-thalassaemia major. Human Reproduction. 20 (7), 1855-1859 (2005).
- Rienzi, L., Ubaldi, F., Anniballo, R., Cerulo, G., Greco, E. Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Human Reproduction. 13 (4), 1014-1019 (1998).
- Wale, P. L., Gardner, D. K. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Human Reproduction Update. 22 (1), 2-22 (2016).
- Rienzi, L., et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Human Reproduction Update. 23 (2), 139-155 (2017).
- Cimadomo, D., et al. Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation. Human Reproduction. , (2018).
- Evans, J., et al. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Human Reproduction Update. 20 (6), 808-821 (2014).
- Capalbo, A., et al. Consistent and reproducible outcomes of blastocyst biopsy and aneuploidy screening across different biopsy practitioners: a multicentre study involving 2586 embryo biopsies. Human Reproduction. 31 (1), 199-208 (2016).
- Cimadomo, D., et al. Failure mode and effects analysis of witnessing protocols for ensuring traceability during PGD/PGS cycles. Reproductive Biomedicine Online. 33 (3), 360-369 (2016).
- Gardner, D. K., Schoolcraft, B., Jansen, R., Mortimer, D. . Towards Reproductive Certainty: Infertility and Genetics Beyond 1999. , 377-388 (1999).
- Cimadomo, D., et al. Inconclusive chromosomal assessment after blastocyst biopsy: prevalence, causative factors and outcomes after re-biopsy and re-vitrification. A multicenter experience. Human Reproduction. , (2018).
- de Boer, K. A., Catt, J. W., Jansen, R. P., Leigh, D., McArthur, S. Moving to blastocyst biopsy for preimplantation genetic diagnosis and single embryo transfer at Sydney IVF. Fertility and Sterility. 82 (2), 295-298 (2004).
- Capalbo, A., Rienzi, L. Mosaicism between trophectoderm and inner cell mass. Fertility and Sterility. 107 (5), 1098-1106 (2017).
- McCoy, R. C., et al. Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development. PLoS Genetics. 11 (10), e1005601 (2015).
- Scott, R. T., Upham, K. M., Forman, E. J., Zhao, T., Treff, N. R. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertility and Sterility. 100 (3), 624-630 (2013).
- Lee, H., et al. Live births after transfer of rebiopsy and revitrification of blastocyst that had “no diagnosis” following trophectoderm biopsy. Fertility and Sterility. 106 (3), e164 (2016).
- Capalbo, A., et al. Diagnostic efficacy of blastocoel fluid and spent media as sources of DNA for preimplantation genetic testing in standard clinical conditions. Fertility and Sterility. 110 (5), 870-879 (2018).
- Hammond, E. R., Shelling, A. N., Cree, L. M. Nuclear and mitochondrial DNA in blastocoele fluid and embryo culture medium: evidence and potential clinical use. Human Reproduction. 31 (8), 1653-1661 (2016).
- Vera-Rodriguez, M., et al. Origin and composition of cell-free DNA in spent medium from human embryo culture during preimplantation development. Human Reproduction. 33 (4), 745-756 (2018).

