Pressure Controlled Ventilation to Induce Acute Lung Injury in Mice
Summary
A murine model for ventilator induced lung injury is an important tool to study an acute lung injury in vivo. Here, we report an easy applicable in situ model for acute lung injury using high-pressure mechanical ventilation to induce acute failure of the lung.
Abstract
Murine models are extensively used to investigate acute injuries of different organs systems (1-34). Acute lung injury (ALI), which occurs with prolonged mechanical ventilation, contributes to morbidity and mortality of critical illness, and studies on novel genetic or pharmacological targets are areas of intense investigation (1-3, 5, 8, 26, 30, 33-36). ALI is defined by the acute onset of the disease, which leads to non-cardiac pulmonary edema and subsequent impairment of pulmonary gas exchange (36). We have developed a murine model of ALI by using a pressure-controlled ventilation to induce ventilator-induced lung injury (2). For this purpose, C57BL/6 mice are anesthetized and a tracheotomy is performed followed by induction of ALI via mechanical ventilation. Mice are ventilated in a pressure-controlled setting with an inspiratory peak pressure of 45 mbar over 1 – 3 hours. As outcome parameters, pulmonary edema (wet-to-dry ratio), bronchoalveolar fluid albumin content, bronchoalveolar fluid and pulmonary tissue myeloperoxidase content and pulmonary gas exchange are assessed (2). Using this technique we could show that it sufficiently induces acute lung inflammation and can distinguish between different treatment groups or genotypes (1-3, 5). Therefore this technique may be helpful for researchers who pursue molecular mechanisms involved in ALI using a genetic approach in mice with gene-targeted deletion.
Protocol
General remarks:
All operations should be performed under an upright dissecting microscope (Olympus, SZX10 with Z-Axis Crank Post with STU2 Stand Boom Stand) and by using a surgical coagulator (11). The experimental groups should be matched as best as possible in age and weight to ensure comparability of the results. Temperature, blood pressure, anesthesia and fluid administration should be stable throughout.
1. Anesthesia and trachea preparation
- Use C57BL/6 mice that are at least 10 weeks old and have a body of 22-25 g. Induce Anesthesia using sodium pentobarbital at a dose 70 mg/kg body weight i.p (6). Maintain anesthesia approximately with 10 mg/kg/h sodium pentobarbital. Be cautious with overdosing since this might significantly lower the blood pressure. Re-dosing of pentobarbital – even after hrs- can lead to severe increases in plasma levels.
- After anesthesia induction, secure mice in a supine position with the upper and lower extremities attached to the table using a tape and a suture fastened to the ankles. Do the same for the head by using the teeth. Sufficient restraining is important for a successful intubation and a well controlled surgery. Prior to surgery, cover the mouse with mineral oil to reduce the risk of mouse hair allergy. In order to ensure that the body temperature remains stable cover the mice with commercially available food wrap.
- Place mice on a temperature-controlled heated table (RT, Effenberg, Munich, Germany) with a rectal thermometer probe attached to thermal feedback controller to maintain body temperature at 37°C.
- Expose the trachea surgically. Dissect lateral and dorsal sides of the trachea of the connective tissue and place two 3.0 silk surgical suture (Harvard apparatus, USA) each 10 cm long underneath the trachea. The sutures should be approximately 1 cm apart.
- Carefully incise the trachea 3 – 4 mm below larynx between two circular cartilages using a McPherson-Vannas Scissors (8 cm, straight blade; World Precision Instruments, USA). Make sure not to cause a bleeding, since this might confound the outcome parameters.
- Perform a tracheal intubation using a blunt polyethylene cannulae (Insyte 22g, Beckton Dickinson, USA). Insert the tip of the polyethylene cannulae in an 85 degree angle into the trachea. Then tilt the cannulae so it is in line with the tracheal lumen. Slowly advance the tube further down the trachea until the tip of cannulae has disappeared in the thorax aperture. Fixate the tube in this position with the two surgical silk suture placed dorsal of the trachea (see 1.4).
2. Technique of ventilator-induced lung injury
- Connect the tube to a ventilator. To induce lung injury we use a pressure controlled ventilation technique by using a Servo 900 C from Siemens (DRE Veterinary, USA). Animals will be ventilated using peak inspiratory pressure of 45 mbar, frequency of 80 breaths/min and a positive end-expiratory pressure of 0-3 mbar with a FiO2 = 1.0. The inspiration to expiration ratio should be 1:1. Despite of the fact that the Servo 900 C is built as ventilator for humans, its use in a pressure controlled ventilator setting works excellent for the ventilation of mice.
- Monitor heart rate with an ECG (e.g. Hewlett Packard, B blingen, Germany). Make sure that the heart rate does not drop below 400. One should see a shift of the heart axis to the right, when mechanical ventilation is instituted as a sign of increased pulmonary artery pressures subsequent to an increase in intrathoracic pressure. If the mouse develops bradycardia, check the temperature and the anesthetic dose/concentration. Xylacin/ Ketamin anesthesia induces a heart heart of 250/ min and is therefore not recommended.
- Apply a proper fluid replacement. An infusion with normal saline 0.1 ml/hour via an arterial or venous catheter should be performed prior to the ventilation to ensure sufficient venous filing. Due to the high ventilation pressures the venous return to the heart is impaired, which might lead to a critical drop of the mean arterial pressure. Also, a saline bolus of 500 μl could be given i.p. prior to surgery.
- Place a catheter in the carotid artery for continuous recording of blood pressure (27). Attach the arm to the body before you start dissecting the artery. The carotid artery is exposed via blunt dissection of the paratracheal muscles. Following further exposure and careful avoidance of any tissue trauma (particular of the vagal nerve), a catheter is inserted into the vessel using two sutures and a small clamp (37). This will expose a longer segment of the artery. Place a ligature at the very end of the carotid artery. Attach a larger clamp to the end of the suture to obtain tension or fixate the suture to the table using tape. Place another suture around the artery and dissect the artery to the very distal end. Here, place a small clamp. Use micro scissors to cut a small diagonal opening into the artery. Hold the opening with a fine forceps (Dumont, WPI) and advance the proper sized catheter with your hands/forceps. Make a knot with your second suture and secure the artery. Loosen the clamp and advance the catheter further. Secure the catheter with several knots and tape. Alternatively the carotid artery catheter can be place at the end of the experiment to collect arterial blood samples for blood gas analysis.
3. Recovery of tissue samples
After 3 hours of mechanical ventilation, samples are collected to assess the extent of lung injury. We recommend collecting brochnoalveolar lavage fluid (BAL), arterial blood and lung tissue.
- Obtain BAL fluid at the end of the experiment. After deepening of anesthesia, flush the tracheal tube with 1 ml phosphate buffered saline (PBS). The fluid should remain in the trachea/lung for three seconds before it is recovery via the connected syringe. The BAL is snap-frozen in liquid nitrogen and stored at -80°C for further analysis. Be aware that the recovered volume could be significantly less than 1 ml.
- Perform blood gas analysis at the end of the experiment. In order to do that, an incision should be made right below the sternum. Hold the sternum with forceps and extent the incision along the rib cage. Next, the diaphragm is incised at the edges and is cut out from the ribs. Now there is an open view into the lower aperture of the thorax. Lift the sternum up with forceps and open the thorax by long cuts at the right and left side (as lateral as possible) so that the complete anterior thorax wall is turned up-wards. This should be done, while the experimental animal is still mechanically ventilated. The left ventricle is punctured using a 27 ½G needle and arterial blood analysis is performed using the i-STAT System (Abbott, USA). If the arterial blood gas analysis should be performed, a BAL cannot be obtained, since this will be a significant confounder to the results.
- Alternatively to method above, arterial blood samples can be collected via the carotid artery catheter. However this should be done before the BAL is obtained.
- Excise the lungs en-bloc by pulling up the heart and cut the trachea. Have a piece of tissue ready to absorb blood, so the surgical site is visible. Pull the heart in the direction of the abdomen and carefully cut along the spine to mobilize all thoracic organs. Cut the aorta, remove the thoracic organs and place them on a clean surgical table.
- Cut away the heart and the major vessel of the tissue sample. Make sure that there is no thymus tissue still attached to the lung. Separate the lungs with a scissor and place in individual tubes and snap-freeze. Store at -80°C for further analysis.
4. Measurement of lung injury
We recommend using the following outcome parameters to assess the extent of lung injury: Perform an albumin ELISA (Bethyl Laboratories, USA) and a myeloperoxidase (MPO) ELISA (Hycult Biotechnology, USA) to assess the extent of barrier dysfunction and the amount of inflammatory cells in the BAL fluid. Perform an MPO ELISA also form the lung tissue. If wet-to-dry ratio is to be measured we do not obtain BAL fluid and the pulmonary circulation is not flushed (see 3.3). Measure the weight of the lungs after excision. Then lungs are lyophilized for 48 h and lung tissue again is measured. Then the wet-to-dry ratio is measured as mg of water per mg of dry tissue (5).
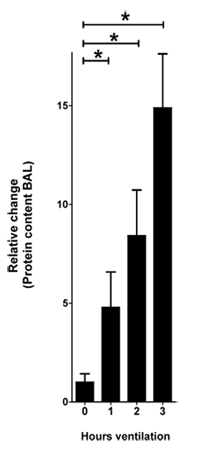
Figure 1. Protein content in the BAL in response to VILI. Mice were anesthetized with pentobarbital, mechanical ventilation
was instituted and mice were ventilated using pressure-controlled settings (inspiratory pressure of 45 mbar, positive end-exspiratory pressure
3 mbar, 100% inspired oxygen concentration). After 0, 1, 2 and 3 hours of ventilation BAL was harvested and the protein content was quantified using a
bicinchoninic acid assay (BCA assay). The relative change of the protein content is shown normalized to 0 hours of ventilation
(n=4 per group, * indicates p < 0.05 compared to control, mean ±SEM)
Discussion
The present study describes a technique of performing ventilator-induced lung injury in mice. This model demonstrates highly reproducible injury due to high pressure ventilation. Investigators who consider studying acute-lung injury in mice may benefit from this model.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The present studies are supported by National Heart, Lung, and Blood Institute Grant R01-HL0921, R01-DK083385 and R01HL098294 to H. K. Eltzschig, the 1K08HL102267-01 to T. Eckle, and Foundation for Anesthesia Education and Research Grants to T. Eckle and H. K. Eltzschig, and American Heart Association Grant to T. Eckle and H. K. Eltzschig and Deutsche Forschungsgemeinschaft (DFG) research fellowship to M. Koeppen.
Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| Sodium Pentobarbital (Fatal Plus) | Vortech Pharmaceutical Ls, Ltd, | V.P.L. 9372 | 4mg/mL in saline |
| Insyte 22 G | Beckton Dickinson | n/a | |
| Suture, silk 4.0 | Harvard Apparatus | 517698 | |
| Suture, Prolene 8.0 | Ethicon, USA | M8739 | reusable |
| Siemens 900°C | DRE Veterinary, USA | # 336 | refurbished |
| dissecting microscope (SZX10 ) | Olympus | n/a | consider generous working distance |
| Heating Table | Rt, Effenberger, Germany | n/a | only and single provider |
| Blood pressure device | Cyber Sense, Inc | BPM02 | |
| I STAT | Abbott | n/a |
References
- Eckle, T., Faigle, M., Grenz, A., Laucher, S., Thompson, L. F., Eltzschig, H. K. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 111, 2024-2035 (2008).
- Eckle, T., Fullbier, L. G. r. e. n. z., A, ., Eltzschig, H. K. Usefulness of pressure-controlled ventilation at high inspiratory pressures to induce acute lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 295, 718-724 (2008).
- Eckle, T., Fullbier, L., Wehrmann, M., Khoury, J., Mittelbronn, M., Ibla, J., Rosenberger, P., Eltzschig, H. K. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 178, 8127-8137 (2007).
- Eckle, T., Grenz, A., Kohler, D., Redel, A., Falk, M., Rolauffs, B., Osswald, H., Kehl, F., Eltzschig, H. K. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 291, 2533-2540 (2006).
- Eckle, T., Grenz, A., Laucher, S., Eltzschig, H. K. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 118, 3301-3315 (2008).
- Eckle, T., Krahn, T., Grenz, A., Kohler, D., Mittelbronn, M., Ledent, C., Jacobson, M. A., Osswald, H., Thompson, L. F., Unertl, K., Eltzschig, H. K. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 115, 1581-1590 (2007).
- Eltzschig, H. K. Adenosine: an old drug newly discovered. Anesthesiology. 111, 904-915 (2009).
- Eltzschig, H. K., Eckle, T., Mager, A., Kuper, N., Karcher, C., Weissmuller, T., Boengler, K., Schulz, R., Robson, S. C., Colgan, S. P. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 99, 1100-1108 (2006).
- Eltzschig, H. K., Ibla, J. C., Furuta, G. T., Leonard, M. O., Jacobson, K. A., Enjyoji, K., Robson, S. C., Colgan, S. P. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 198, 783-796 (2003).
- Eltzschig, H. K., Kohler, D., Eckle, T., Kong, T., Robson, S. C., Colgan, S. P. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 113, 224-232 (2009).
- Frick, J. S., MacManus, C. F., Scully, M., Glover, L. E., Eltzschig, H. K., Colgan, S. P. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 182, 4957-4964 (2009).
- Grenz, A., Eckle, T., Zhang, H., Huang, D. Y., Wehrmann, M., Kohle, C., Unertl, K., Osswald, H., Eltzschig, H. K. Use of a hanging-weight system for isolated renal artery occlusion during ischemic preconditioning in mice. Am J Physiol Renal Physiol. 292, 475-485 (2007).
- Grenz, A., Osswald, H., Eckle, T., Yang, D., Zhang, H., Tran, Z. V., Klingel, K., Ravid, K., Eltzschig, H. K. The Reno-Vascular A2B Adenosine Receptor Protects the Kidney from Ischemia. PLoS Medicine. 5, e137-e137 (2008).
- Grenz, A., Zhang, H., Eckle, T., Mittelbronn, M., Wehrmann, M., Kohle, C., Kloor, D., Thompson, L. F., Osswald, H., Eltzschig, H. K. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 18, 833-845 (2007).
- Grenz, A., Zhang, H., Hermes, M., Eckle, T., Klingel, K., Huang, D. Y., Muller, C. E., Robson, S. C., Osswald, H., Eltzschig, H. K. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 21, 2863-2873 (2007).
- Grenz, A., Zhang, H., Weingart, J., von Wietersheim, S., Eckle, T., Schnermann, J. B., Kohle, C., Kloor, D., Gleiter, C. H., Vallon, V., Eltzschig, H. K., Osswald, H. Lack of effect of extracellular adenosine generation and signalling on renal erythropoietin secretion during hypoxia. Am J Physiol Renal Physiol. , (2007).
- Haeberle, H. A., Durrstein, C., Rosenberger, P., Hosakote, Y. M., Kuhlicke, J., Kempf, V. A., Garofalo, R. P., Eltzschig, H. K. Oxygen-independent stabilization of hypoxia inducible factor (HIF)-1 during RSV infection. PLoS ONE. 3, e3352-e3352 (2008).
- Hart, M. L., Gorzolla, I. C., Schittenhelm, J., Robson, S. C., Eltzschig, H. K. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 184, 4017-4024 (2010).
- Hart, M. L., Henn, M., Kohler, D., Kloor, D., Mittelbronn, M., Gorzolla, I. C., Stahl, G. L., Eltzschig, H. K. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J. 22, 2784-2797 (2008).
- Hart, M. L., Jacobi, B., Schittenhelm, J., Henn, M., Eltzschig, H. K. Cutting Edge: A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol. 182, 3965-3968 (2009).
- Hart, M. L., Kohler, D., Eckle, T., Kloor, D., Stahl, G. L., Eltzschig, H. K. Direct treatment of mouse or human blood with soluble 5′-nucleotidase inhibits platelet aggregation. Arterioscler Thromb Vasc Biol. 28, 1477-1483 (2008).
- Hart, M. L., Much, C., Gorzolla, I. C., Schittenhelm, J., Kloor, D., Stahl, G. L., Eltzschig, H. K. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 135, 1739-1750 (2008).
- Hart, M. L., Much, C., Kohler, D., Schittenhelm, J., Gorzolla, I. C., Stahl, G. L., Eltzschig, H. K. Use of a hanging-weight system for liver ischemic preconditioning in mice. Am J Physiol Gastrointest Liver Physiol. 294, 1431-1440 (2008).
- Hartmann, H., Eltzschig, H. K., Wurz, H., Hantke, K., Rakin, A., Yazdi, A. S., Matteoli, G., Bohn, E., Autenrieth, I. B., Karhausen, J., Neumann, D., Colgan, S. P., Kempf, V. A. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology. 134, 756-767 (2008).
- Heinzelmann, F., Jendrossek, V., Lauber, K., Nowak, K., Eldh, T., Boras, R., Handrick, R., Henkel, M., Martin, C., Uhlig, S., Kohler, D., Eltzschig, H. K., Wehrmann, M., Budach, W., Belka, C. Irradiation-induced pneumonitis mediated by the CD95/CD95-ligand system. J Natl Cancer Inst. 98, 1248-1251 (2006).
- Koeppen, M., Eckle, T., Eltzschig, H. K. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One. 4, e6784-e6784 (2009).
- Kohler, D., Eckle, T., Faigle, M., Grenz, A., Mittelbronn, M., Laucher, S., Hart, M. L., Robson, S. C., Muller, C. E., Eltzschig, H. K. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 116, 1784-1794 (2007).
- Kuhlicke, J., Frick, J. S., Morote-Garcia, J. C., Rosenberger, P., Eltzschig, H. K. Hypoxia Inducible Factor (HIF)-1 Coordinates Induction of Toll-Like Receptors TLR2 and TLR6 during Hypoxia. PLoS ONE. 2, e1364-e1364 (2007).
- Morote-Garcia, J. C., Rosenberger, P., Kuhlicke, J., Eltzschig, H. K. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 111, 5571-5580 (2008).
- Morote-Garcia, J. C., Rosenberger, P., Nivillac, N. M., Coe, I. R., Eltzschig, H. K. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 136, 607-618 (2009).
- Reutershan, J., Vollmer, I., Stark, S., Wagner, R., Ngamsri, K. C., Eltzschig, H. K. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 23, 473-482 (2009).
- Schingnitz, U., Hartmann, K., Macmanus, C. F., Eckle, T., Zug, S., Colgan, S. P., Eltzschig, H. K. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 184, 5271-5279 (2010).
- Thompson, L. F., Eltzschig, H. K., Ibla, J. C., Van De Wiele, C. J., Resta, R., Morote-Garcia, J. C., Colgan, S. P. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 200, 1395-1405 (2004).
- Aherne, C. M., Kewley, E. M., Eltzschig, H. K. The resurgence of A2B adenosine receptor signaling. Biochim Biophys Acta. , (2010).
- Eckle, T., Koeppen, M., Eltzschig, H. K. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda). 24, 298-306 (2009).
- Koeppen, M., Feil, R., Siegl, D., Feil, S., Hofmann, F., Pohl, U., de Wit, C. cGMP-dependent protein kinase mediates NO- but not acetylcholine-induced dilations in resistance vessels in vivo. Hypertension. 44, 952-955 (2004).

