Murine Model of Allergen Induced Asthma
Summary
Experimental mouse models of allergic asthma offer new possibilities for studying disease pathogenesis and developing new therapeutics. These models are well suited to measuring factors governing the allergic immune response, airway inflammation, and pulmonary pathophysiology.
Abstract
Asthma is a major cause of morbidity and mortality, affecting some 300 million people throughout the world.1 More than 8% of the US population has asthma, with the prevalence increasing.2 As with other diseases, animal models of allergic airway disease greatly facilitate understanding of the underlying pathophysiology, help identify potential therapeutic targets, and allow preclinical testing of possible new therapies. Models of allergic airway disease have been developed in several animal species, but murine models are particularly attractive due to the low cost, ready availability, and well-characterized immune systems of these animals.3 Availability of a variety of transgenic strains further increases the attractiveness of these models.4 Here we describe two murine models of allergic airway disease, both employing ovalbumin as the antigen. Following initial sensitization by intraperitoneal injection, one model delivers the antigen challenge by nebulization, the other by intratracheal delivery. These two models offer complementary advantages, with each mimicking the major features of human asthma.5
The major features of acute asthma include an exaggerated airway response to stimuli such as methacholine (airway hyperresponsiveness; AHR) and eosinophil-rich airway inflammation. These are also prominent effects of allergen challenge in our murine models,5,6 and we describe techniques for measuring them and thus evaluating the effects of experimental manipulation. Specifically, we describe both invasive7 and non-invasive8 techniques for measuring airway hyperresponsiveness as well as methods for assessing infiltration of inflammatory cells into the airways and the lung. Airway inflammatory cells are collected by bronchoalveolar lavage while lung histopathology is used to assess markers of inflammation throughout the organ. These techniques provide powerful tools for studying asthma in ways that would not be possible in humans.
Protocol
I. Allergen Sensitization and Challenge (see Figure 1)
A. For Intratracheal Challenge
- For initial sensitization, inject male or female C57BL/6 or BALB/c mice (6-8 weeks old) intraperitoneally on day 0 and again on day 7 with 20 μg of ovalbumin (OVA; Sigma-Aldrich, St. Louis, MO) emulsified in 0.2 ml of sterile phosphate buffered saline (PBS) containing 2 mg of aluminum hydroxide (Sigma-Aldrich) or with 2 mg aluminum hydroxide in 0.2 ml of sterile PBS as control.
- Challenge with antigen as appropriate (e.g., on days 14, 16, 18, and 20). Challenge procedure follows.
- Anesthetize mouse with an intraperitoneal (i.p.) injection of a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg). Ensure the mouse is fully anesthetized for at least 10 min.
- Angle operation surface at 45 degrees or greater. Place mouse on this surface keeping ventral side upward and head at top.
- Hook thread under front incisors to hold head back. Level the paws with one another to ensure the trachea is straight and use label tape to hold legs. Soak surgical site with 70% EtOH and swab.
- Apply bupivicaine (0.1 to 0.2 ml of 0.25% solution) topically at the incision site.
- Nip skin on the throat with forceps and pull gently outward. Make a small vertical incision with surgical scissors. Minimize size of incision.
- Prepare a 1 ml syringe with 50 μl of PBS or 0.1% OVA in PBS and insert it into a repetitive pipette (Tridak Stepper, Torrington, CT). Take the pipette in one hand and use the other to hold the tissue back with the tweezers and expose the trachea.
- Holding the syringe as parallel to the trachea as possible, insert needle through trachea wall and inject the solution.
- Maintain mouse in a vertical orientation following injection to allow time for solution to settle in the lungs.
- Gently and sterilely close wound area with tweezers and seal with suture.
- Place mouse sternum-down on a heating pad and allow it to recover until fully ambulatory. Following recovery, return the mouse to the animal care facility and monitor it daily for signs of seroma, inflammation or infection, and wound dehiscence until the wound is fully healed.
B. For Challenge by Nebulization
- Sensitize mice on day 0 by intraperitoneal injection of 20 μg of OVA (Sigma-Aldrich) emulsified in 0.2 ml of sterile PBS containing 2 mg of aluminum hydroxide (Sigma-Aldrich) or with 2 mg aluminum hydroxide in 0.2 ml of sterile PBS as control.
- On day 14, boost sensitization by i.p. injection as described above.
- On the 21st, 22nd, 23rd, 24th and 25th day after initial sensitization, challenge mice by exposure for 30 min to nebulized 1% OVA or PBS alone delivered via an ultrasonic nebulizer (Buxco Research Systems, Wilmington, NC).
- Place mice in main chamber of WBP; acclimatize them within the plethysmography chamber for at least 10 min.
- Place 1 ml of 0.1% OVA in sterile PBS or sterile PBS alone, as described in steps 4-6 below, via a nebulizer cup. Nebulize for 30 min.
- Remove nebulizer cup and discard any remaining solution.
- Nebulization can be performed on all mice simultaneously by using a nebulization chamber.
II. Determination of Airway Hyperresponsiveness to Methacholine
A. Noninvasive Measurement of Airway Hyperresponsiveness by Whole-body Plethysmography (WBP; Buxco Research Systems, Wilmington, NC)
- Allow the powdered methacholine bottle to warm to room temperature before opening (methacholine is very hygroscopic and will form useless clumps if allowed to absorb water). Prepare a 200 mg/ml stock solution in sterile PBS, then make serial 2-fold dilutions (e.g., 100, 50, 25, 12.5 and 6.25 mg/ml). Keep solutions cold.
- Set up equipment as follows: connect main inlet of WBP to nebulizer, bias-flow inlet to air-pump, and WBP outlet to gas trap using tight-fitting rubber tubing. Attach pressure transducer to bridge the outlets of the main and reference chambers of the WBP. Connect pressure transducer to preamplifier with the cables provided, and connect preamplifier to PC using specific data-acquisition card.
- Calibrate the preamplifier using the software according to manufacturer’s recommendations.
- Place mice in main chamber of WBP; acclimatize them within the plethysmography chamber for at least 10 min, then record baseline readings (Penhbase) for 3 min.
- Place 1 ml of sterile PBS in the nebulizer cup. Nebulize for 2 min and then monitor respiratory variables for an additional 6 min during the drying phase. Remove nebulizer cup and discard any remaining PBS.
- Place 1 ml of 6.25 mg/ml methacholine in the nebulizer cup and repeat nebulization for 2 min plus a 6-min monitoring cycle.
- Repeat measurement with 12.5, 25, 50 and 100 mg/ml methacholine, using the same 2-min nebulization period and 6-min monitoring cycle.
- Remove the mice from the chambers and return them to their cages.
- Refill nebulizer cup with 1 ml sterile PBS and run another sequence to flush the tubing.
- Shut down the air flows, disassemble, and wipe clean all chambers before running a second set of animals.
B. Invasive Measurement of Airway Responsiveness by Computer-controlled Ventilator (flexiVent; SCIREQ Inc., Montreal, Canada)
- Weigh the mouse and anesthetize by intraperitoneal (i.p.) injection of 60 mg per kg body weight pentobarbital sodium.
- Following adequate anesthesia, position the mice ventro-dorsally for tracheostomy.
- Disinfect the neck skin with 70% ethanol. Apply bupivicaine (0.1 to 0.2 ml of 0.25% solution) topically at the incision site. Incise and open the neck skin. Separate the neck muscles and expose the trachea.
- Make a 1- to 2-mm incision in the trachea with fine scissors (be certain not to sever the trachea) and insert the tracheal tube cautiously. Tie a suture around the trachea to prevent an air leak.
- Lay the mouse in the body plethysmograph chamber and connect the inserted tracheal tube to the ventilator.
- Start mechanical ventilation. Set appropriate respiratory rate and tidal/stroke volume (150 strokes/min and 200 μl, respectively for a 20-g mouse). Be sure that the thorax is moving in synchrony with the ventilator. If the mouse is “fighting” with the ventilator (self-respiration), inject more anesthetic and wait for the synchronization.
- Following baseline measurements, maintain mice under baseline ventilation for another 3 min, and then take a 2nd set of impedance measurements. This 2nd set of baseline measurements is used to calculate the mean baseline values.
- Deliver PBS or methacholine (MCh) challenges (6.25, 12.5, 25, 50 and 100 mg/ml) by channeling inspiratory flow from the ventilator through an ultrasonic nebulizer.
- Following each challenge with MCh (6.25, 12.5, 25, 50 and 100 mg/ml), return the piston to delivering a Vt of 10 ml/kg at 120 breaths/min and take impedance measurements.
III. Measurement of Cellular Infiltration into the Airspace
A. Perform Bronchoalveolar Lavage (BAL)
- After measuring the AHR, euthanize mice with CO2, and position each mouse on its back on the surgical pad.
- Soak the area with 70% EtOH.
- Beginning at the lower abdomen, cut open the abdominal cavity and remove skin/upper muscle, moving upwards toward the ribs.
- Once the ribs are visible, use scissors to carefully puncture the diaphragm. Lungs should collapse away from the diaphragm. Be especially careful not to nick the lungs or heart.
- Cut away the ribcage to fully expose the lungs/heart (avoid cutting any major blood vessels to keep blood from filling the site).
- Using a 1 ml syringe with a 27 gauge needle (BD syringes, Franklin Lakes, NJ), puncture the heart ventricles and slowly and carefully pull back the syringe to collect the blood. Take care to avoid collapsing the heart.
- Collect the serum from this blood using standard protocol. Store at -70 °C until use.
- Cut away skin and tissue from the throat until the trachea is revealed. Clear away sufficient tissue to work easily within the field (again, avoid cutting any major blood vessels).
- Using curved scissors, cut under the trachea to clear a path.
- Pass the point of a curved forceps under the trachea and grasp the end of a piece of suture. Draw the suture thread under the trachea.
- Tie a loose half-knot about the trachea, low in the throat.
- Carefully cut a notch, sufficient in size for the cannula, above the suture thread.
- Carefully insert the cannula into the hole and down the trachea past the point of the suture thread. Gently press forward until the cannula emerges just at the entrance to the lungs (too far: puncture lungs; too short: collapse trachea when attempting to recover BAL).
- Tighten suture thread and complete knot to seal trachea around cannula.
- Lock syringe (containing 1 ml PBS) on the cannula, and gently press the fluid into the lung. Lung lobes should individually inflate slowly. Do not over-fill. For a full grown mouse 0.9-1.0 ml is the absolute maximum. 0.8 ml may be safer. Lock syringe loosely to cannula, otherwise it is likely to cause damage when attempting to disengage.
- Withdraw fluid from lungs. If resistance is encountered (tissue sucked into cannula), press cannula slowly further into the lung and resume removing. Also try rotating the cannula in place. If all else fails, withdraw the cannula part way; the trachea is much more likely to collapse in this case.
- Detach syringe from cannula, deposit BAL fluid in container, and repeat 2 times with fresh PBS solution.
- Keep the BAL solution on ice until spun down.
- Use the BAL fluid and serum to measure the OVA specific IgE using commercially available mouse IgE ELISA kits (MD Bioproducts, St. Paul, MN).
B. Count Cells and Determine Differentials
- Centrifuge the BAL fluid 5 min at ~600 × g, 4 °C.
- Resuspend the cell pellet gently in PBS and keep on ice.
- Load a standard Neubauer hemacytometer with the diluted cell suspension and count the cells.
- Remove aliquots of 2 × 104 cells in 10 to 40 μl volume for cytospins. Dilute cells if necessary.
- For cytospins, mix 2 × 104 cells, 130 μl PBS and 10 μl FBS. Add entire cell mixture to double cytospin funnel and centrifuge 10 min at 700 rpm, using double cytoslides for duplicate samples.
- Allow the slides to dry at room temperature for 1 h prior to staining.
- Stain the slides by using Diff-Quick stain (Siemens, Newark, DE).
IV. Representative Results
Excessive airway constriction following provocative stimuli is a prominent feature of clinical asthma. We describe two methods for measuring such airway hyperresponsiveness to methacholine in OVA-sensitized and challenged mice: Whole-body plethysmography (Figure 2) and forced oscillation using the flexiVent system (Figure 3). Both methods demonstrate that OVA sensitization and challenge produces airway hyperresponsiveness in mice.
Eosinophil-rich airway inflammation is another prominent feature of both clinical asthma and allergic airway disease in mice. As shown in Figure 4, OVA sensitization and challenge greatly increases the total number of cells that can be recovered from the airways by BAL. The numbers of eosinophils and, to a lesser extent, neutrophils are especially increased.
Evidence indicates that allergic airway disease results from overproduction of IgE antibodies to sensitizing antigens. Sensitization and challenge with OVA using the protocols we describe increases IgE levels in both serum and BAL fluid of treated mice (Figure 5).
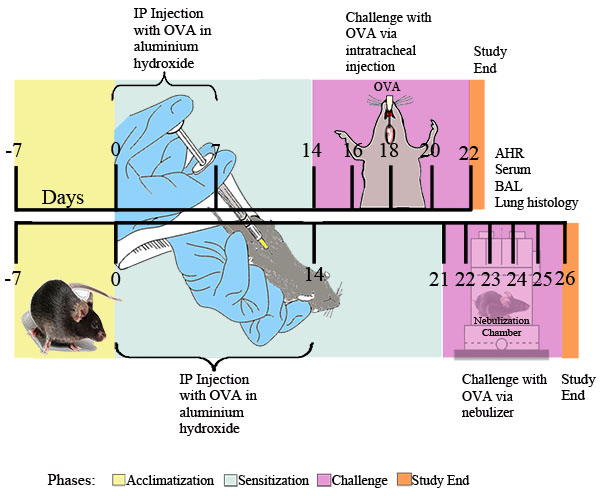
Figure 1. Experimental schema for OVA-induced allergic asthma. Mice were sensitized twice i.p. with 20 μg of OVA emulsified in 2 mg of aluminum hydroxide in 0.2 ml of sterile PBS, or 2 mg of aluminum hydroxide in 0.2 ml of sterile PBS alone, followed at the indicated time points by i.t. challenge with 0.1% OVA or sterile PBS solution or by daily exposure for 30 minutes to nebulized 1% OVA in PBS or PBS alone delivered via an ultrasonic nebulizer (Buxco). Twenty-four hours after the final OVA exposure, airway responsiveness was determined. Subsequently, BAL fluid, blood samples, lung cells, and tissues were collected for further analysis.
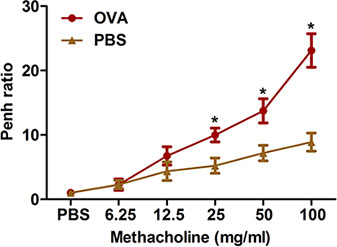
Figure 2. Assessment of allergen-induced airway hyperresponsiveness by a noninvasive method. Mice (n=4/group) were sensitized and challenged with OVA. Twenty-four hours following the last challenge, airway hyperresponsiveness to inhaled methacholine was determined using whole-body plethysmography as described in the protocol. Penh was determined and expressed as Penh ratio (average Penh over the 8-min time interval with methacholine divided by the average Penh over the 8-min interval with PBS). *, P < 0.05 vs. PBS.
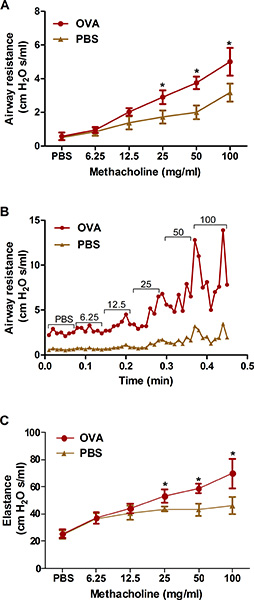
Figure 3. Assessment of allergen-induced airway hyperresponsiveness by an invasive method (forced oscillation). Mice (n=4/group) were sensitized and challenged with OVA. Twenty-four hours following the last challenge, airway hyperresponsiveness to increasing concentrations of inhaled methacholine was determined by the forced oscillation (flexiVent) method as described in the protocol. A, B) Airway resistance; C) Lung elastance. *, P < 0.05 vs. PBS.
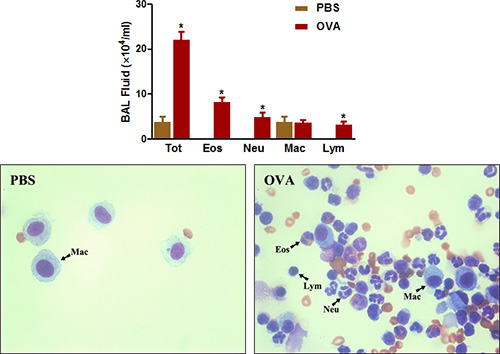
Figure 4. BAL fluid cell count. Mice (n=4/group) were sensitized and challenged with OVA. Twenty-four hours following the last challenge, (Top) BAL cells were collected and total cells were counted as described in the protocol. (Bottom) Cytospin slides were prepared and stained with Diff-Quick. Tot = total cells; Eos = eosinophils; Neu = neutrophils; Mac = macrophages; Lym = lymphocytes. *, P < 0.05 vs. PBS.
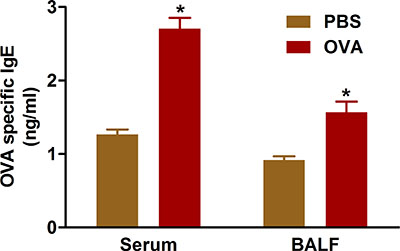
Figure 5. OVA-specific IgE. Mice (n=4/group) were sensitized and challenged with OVA. Twenty-four hours following the last challenge, IgE was measured in BAL fluid and in serum from the blood collected by cardiac puncture as described in the protocol. *, P < 0.05 vs. PBS.
Discussion
Animal models of allergic airway disease provide important tools for studies relevant to clinical asthma. A number of different models, employing varying species and antigens, have been developed. The mouse, an attractive and frequently used laboratory species, also offers a number of advantages for models of allergic airway disease.9,10 Although such models do not mimic asthma in every respect,11 with aspects of chronic disease being particularly difficult to reproduce,12,13 we confirm here that many of the major features are reproduced. We also show that, as in human asthma, these features are associated with increases in antigen-specific IgE in both serum and BAL fluid. We present two murine models, both employing OVA as the antigen but utilizing different challenge techniques. Intratracheal administration is somewhat complex and time-consuming, but offers the advantage of delivering a known quantity of antigen directly to the lung. It is also possible that this method delivers the antigen more deeply into the lung than do alternatives. We describe an invasive method for intratracheal delivery, but antigen can also be delivered intratracheally via a tube or cannula inserted via the oral cavity. This method is described elsewhere in JoVE.14 Nebulization is simpler and more directly mimics the usual route of human exposure. Quantitation is more variable, however, and delivery to the lung may be less efficient.15 Indeed, it is likely that a sizable but unknown fraction of the dose is deposited in the upper airways. A third alternative, not described here, is intranasal delivery. Again, it is likely that a sizable fraction of the dose will be deposited in the upper airways.
Although our demonstration is performed using C57BL/6 mice, evidence suggests that certain other strains may give more robust results on specific endpoints. In comparisons of C57BL/6 with BALB/c16 or with both BALB/c and FVB/NJ17 mice, the C57BL/6 mice showed the smallest increase in AHR. On the other hand, C57BL/6 mice showed greater increases in eosinophilia in one study16 but not in the other.17 Cytokine elevations were both strain- and cytokine-specific. The preferred strain may therefore depend on the endpoint deemed most important.
Two main features of allergic airway disease are commonly chosen for assessment in order to determine the effects of experimental manipulations: AHR and extent of airway inflammation. AHR may be measured either by whole-body plethysmography (WBP) or by forced oscillation. In both cases, methacholine is used as the provocative challenge. WBP is a functional in vivo measurement that allows analysis of airway reactivity on conscious, freely moving or minimally restrained mice without invasive surgery and anesthesia. Airway responsiveness is expressed using the “enhanced pause” (Penh) as a parameter of altered airway function. Penh is an empirical parameter that reflects changes in the box flow waveform from both inspiration and expiration and combines it with the comparison of early and late expiratory box flow. WBP has several potential advantages compared to invasive means for measuring lung resistance, since it is technically less demanding and allows measurements of airway responsiveness to aerosolized stimulants. This method minimizes both effects of psychological stress and animal preparation time, making it ideal for repeated measurements (examination of the same animal at different time points) and measurement over long periods of time (>24 hr), during which a variety of aerosols can be administered in a controlled and repeatable manner. The results correlate strongly with those of the invasive methods, but it is faster and easier.
Two alternative noninvasive methods provide measurements that are said to be more directly related to airway constriction. In double-chamber plethysmography the mouse is forced to stick its head through a hole in the front of the thoracoabdominal chamber.18 A nasal chamber is then fitted to the front of the thoracoabdominal chamber and a head-hole is cut in the sealing latex film between the chambers. This hole is precisely sized to provide an air-tight seal between the chambers without restricting respiratory airflow. Plethysmographic measurements are then taken in both chambers and the delay between nasal and thoracoabdominal flows are used to calculate the specific airway resistance (sRaw). Head-out plethysmography is similar except that air flows freely in the nasal chamber and no plethysmographic measurements are made there.19 The primary calculated parameter is flow rate at midexpiratory phase (EF50). Both techniques, like WBP, are suitable for repeated measurements over a number of hours, permitting assessment of both early and late responses. However, both require the animals to be restrained, which can produce erratic results unless the animals are accustomed to the apparatus over the course of several days. It can also be difficult to assure an effective air-tight seal, which is essential in both cases. Furthermore, since both Penh and sRaw have been shown to correlate strongly with invasive measurements, and Penh and sRaw have been shown to correlate directly in guinea pigs,20 it can be questioned whether the additional information obtained justifies the increased experimental difficulty.
In the forced oscillation method, deeply anesthetized mice are tracheotomized and a mechanical ventilator is connected to the tube. Pressure-flow measurements as the ventilator inflates and deflates the lung then allow direct measurement of pulmonary resistance and dynamic compliance. This provides information about airway mechanics that is not available with WBP. Forced oscillation is technically more difficult, however, and is typically a terminal procedure. As experimental endpoints, the two procedures generally give similar results.19
The other prominent feature of clinical asthma commonly used to assess the effect of experimental manipulations in murine allergic airway disease is eosinophilic inflammation. Although other aspects of inflammation may be measured as well, this most commonly involves determination of the extent of inflammatory cell (especially eosinophil) infiltration into the airways, the lung, or both. The number and type of cells in the airways is determined in BAL fluid: First total cell number is determined by counting in a hemacytometer, and then cell type is determined by differential staining following cytospin. Although BAL fluid collection can in principle be performed in living mice, as it is in humans, it is more usually and conveniently performed following euthanasia. Eosinophil infiltration into the lungs is assessed by lung excision followed by standard histopathological techniques.
In some instances it may also be useful to assess the extent to which antigen sensitization and challenge stimulates goblet (mucus-producing) cell proliferation. This is easily accomplished by staining the lung sections with periodic acid-Schiff’s, which is specific for mucus. Antigen-specific IgE may also be measured in BAL fluid and serum using readily available kits. IgE measurement may be important in settings where the degree of sensitization, as distinct from the end-organ response, is a crucial parameter. There are a wide variety of other measurements, including cytokines and T-cell responses, that are valuable adjuncts to the study of allergic airway disease in animal models that we have not described here. It is the wide variety of relevant responses that antigen sensitization and challenge can elicit that render such models valuable in study of this disease.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH Grant HL093196 (R.C.R.) and the Atlanta Research and Education Foundation (AREF).
Materials
| Material Name | Company | Catalogue Number | Comments |
| Ovalbumin | Sigma-Aldrich St. Louis, MO |
A5503 | |
| Aluminum hydroxide | Sigma-Aldrich | 239186 | |
| Acetyl-β-methylcholine chloride | Sigma-Aldrich | A2251 | |
| Pentobarbital sodium salt | Sigma-Aldrich | P3761 | |
| Whole body plethysmography (WBP) system |
Buxco Research Systems Wilmington, NC |
http://www.buxco.com | |
| FlexiVent | SCIREQ, Inc. Montreal, Canada |
http://www.scireq.com | |
| Light microscope | Leica Microsystems, Inc. Buffalo Grove, IL |
||
| Cytospin 4 | Thermo Scientific Asheville, NC |
||
| Diff-Quick stain | Siemens Newark, DE |
B4132-1A | |
| Repetitive pipette | Tridak Torrington, CT |
STP4001-0025 |
References
- Braman, S. S. The global burden of asthma. Chest. 130, 4S-12S (2006).
- Akinbami, L. J., Mooman, J. E., Liu, X. Asthma Prevalence, Health Care Use, and Mortality: 2005-2009. National Health Statistics Reports. 32, 2005-2009 (2011).
- Bates, J. H., Rincon, M., Irvin, C. G. Animal models of asthma. Am. J. Physiol. Lung. Cell. Mol. Physiol. 297, 401-410 (2009).
- Drazen, J. M., Finn, P. W., De Sanctis, G. T. Mouse models of airway responsiveness: physiological basis of observed outcomes and analysis of selected examples using these outcome indicators. Annu. Rev. Physiol. 61, 593-625 (1999).
- Epstein, M. M. Do mouse models of allergic asthma mimic clinical disease. Int. Arch. Allergy Immunol. 133, 84-100 (2004).
- Blyth, D. I., Pedrick, M. S., Savage, T. J., Hessel, E. M., Fattah, D. Lung inflammation and epithelial changes in a murine model of atopic asthma. Am. J. Respir. Cell Mol. Biol. 14, 425-438 (1996).
- Martin, T. R., Gerard, N. P., Galli, S. J., Drazen, J. M. Pulmonary responses to bronchoconstrictor agonists in the mouse. J. Appl. Physiol. 64, 2318-2323 (1988).
- Hamelmann, E. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156, 766-775 (1997).
- Gelfand, E. W. Pro: mice are a good model of human airway disease. Am. J. Respir. Crit. Care Med. 166, 5-8 (2002).
- Shapiro, S. D. Animal models of asthma: Pro: Allergic avoidance of animal (model[s]) is not an option. Am. J. Respir. Crit. Care Med. 174, 1171-1173 (2006).
- Zosky, G. R. Ovalbumin-sensitized mice are good models for airway hyperresponsiveness but not acute physiological responses to allergen inhalation. Clin. Exp. Allergy. 38, 829-838 (2008).
- Nials, A. T., Uddin, S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis. Model. Mech. 1, 213-220 (2008).
- Wenzel, S., Holgate, S. T. The mouse trap: It still yields few answers in asthma. Am. J. Respir. Crit. Care Med. 174, 1173-1178 (2006).
- Rayamajhi, M. Non-surgical Intratracheal Instillation of Mice with Analysis of Lungs and Lung Draining Lymph Nodes by Flow Cytometry. J. Vis. Exp. (51), e2702 (2011).
- Swedin, L. Comparison of aerosol and intranasal challenge in a mouse model of allergic airway inflammation and hyperresponsiveness. Int. Arch. Allergy Immunol. 153, 249-258 (2010).
- Gueders, M. M. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm. Res. 58, 845-854 (2009).
- Zhu, W., Gilmour, M. I. Comparison of allergic lung disease in three mouse strains after systemic or mucosal sensitization with ovalbumin antigen. Immunogenetics. 61, 199-207 (2009).
- Flandre, T. D., Leroy, P. L., Desmecht, D. J. Effect of somatic growth, strain, and sex on double-chamber plethysmographic respiratory function values in healthy mice. J. Appl. Physiol. 94, 1129-1136 (2003).
- Hoymann, H. G. Invasive and noninvasive lung function measurements in rodents. J. Pharmacol. Toxicol. Methods. 55, 16-26 (2007).
- Chong, B. T., Agrawal, D. K., Romero, F. A., Townley, R. G. Measurement of bronchoconstriction using whole-body plethysmograph: comparison of freely moving versus restrained guinea pigs. J. Pharmacol. Toxicol. Methods. 39, 163-168 (1998).

