The Polyvinyl Alcohol Sponge Model Implantation
Summary
A useful tool to analyze the effects of drugs, growth factors, and/or manipulated cells in an animal model of wound repair is described. This technique utilizes the properties of a polyvinyl alcohol (PVA) sponge to deliver and contain the desired treatment and also provide a platform to be excised and analyzed.
Abstract
Wound healing is a complicated, multistep process involving many cell types, growth factors and compounds1-3. Because of this complexity, wound healing studies are most comprehensive when carried out in vivo. There are many in vivo models available to study acute wound healing, including incisional, excisional, dead space, and burns. Dead space models are artificial, porous implants which are used to study tissue formation and the effects of substances on the wound. Some of the commonly used dead space models include polyvinyl alcohol (PVA) sponges, steel wire mesh cylinders, expanded polytetrafluoroethylene (ePTFE) material, and the Cellstick1,2.
Each dead space model has its own limitations based on its material’s composition and implantation methods. The steel wire mesh cylinder model has a lag phase of infiltration after implantation and requires a long amount of time before granulation tissue formation begins1. Later stages of wound healing are best analyzed using the ePTFE model1,4. The Cellstick is a cellulose sponge inside a silicon tube model which is typically used for studying human surgery wounds and wound fluid2. The PVA sponge is limited to acute studies because with time it begins to provoke a foreign body response which causes a giant cell reaction in the animal5. Unlike other materials, PVA sponges are easy to insert and remove, made of inert and non-biodegradable materials and yet are soft enough to be sectioned for histological analysis2,5.
In wound healing the PVA sponge is very useful for analyzing granulation tissue formation, collagen deposition, wound fluid composition, and the effects of substances on the healing process1,2,5. In addition to its use in studying a wide array of attributes of wound healing, the PVA sponge has also been used in many other types of studies. It has been utilized to investigate tumor angiogenesis, drug delivery and stem cell survival and engraftment1,2,6,7. With its great alterability, prior extensive use, and reproducible results, the PVA sponge is an ideal model for many studies1,2.
Here, we will describe the preparation, implantation and retrieval of PVA sponge disks (Figure 1) in a mouse model of wound healing.
Protocol
1. Sponge Preparation
- Hydrate sponges by stirring overnight in 0.9% (w/v) aqueous sodium chloride solution.
- Sterilize hydrated sponges by autoclaving them. For 75 mL, autoclave for 25 minutes at 121 °C.
Note: Loading sponges with a treatment as described below is optional.
- In a sterile hood with sterile instruments, pick up one sponge from the solution using a tweezers. Squeeze the sponge with the tweezers and use a vacuum tip to remove as much solution as possible from the sponge. Place the wrung out sponge in a tissue culture dish, being careful to leave space between sponges. Multiple well plates can be used to separate different treatments.
- Pipette the treatment solution directly onto the center of a sponge. Press down gently on the sponge with the pipette tip after the solution has been placed on the sponge; this helps the sponge absorb the solution. Note: The 6mm diameter by 2.75 mm thick sponges that we employ are able to hold a maximum of 25 μL.
- Once all sponges are loaded, the plate they are in can be stored on ice until they are implanted in the mouse.
2. Surgical Procedure
- Appropriately anesthetize animal and administer pre-emptive analgesic. The entire procedure will last approximately 20 minutes per animal. For a 30 g mouse 1.5 liters per minute of oxygen with 1.5% Isoflurane is used to induce and maintain anesthesia. When animal is no longer responsive to the pinch reflex test, place in nose cone in supine position. Use an electric shaver to shave a 1 in. by 1 in. area on the lower ventral side below the rib cage. Wipe clean of cut hair.
- Sterilize the shaved area by cleaning with betadine followed by 70% alcohol. Repeat this betadine-alcohol cleansing three times.
- While holding the skin taught, use the scalpel to make a vertical incision 1.5-2 times the diameter of the sponges to be inserted, being cautious not to cut through the body cavity.
- Use a tweezers to hold the edge of the skin and slowly begin separating the skin from the muscle on both sides of the incision using Metzembaum scissors. The pocket should go across the width of the mouse and from the rib cage to the hind legs.
- Use a tweezers to pick up the first sponge to be placed in the animal by pinching it from the sides. Transfer the sponge to a straight tweezers so that it is held from the top and bottom.
- Use tweezers to hold the edge of the skin and lift it so that the sponge can be inserted. Slowly insert the sponge trying to avoid touching the skin or the muscle underneath as the sponge will be difficult to move once it has touched tissue. Repeat for all sponges.
- Once all sponges have been inserted, place two or three sutures to close the incision. Use a pair of tweezers to hold both sides of the incision together and thread the suture through both layers of skin. Tighten the first throw just enough to close the skin edges. Finish the square knot with one more throw from the other direction, this time pulling the knot tight. Then tie two more square knots to finish the suture (Figure 2). Place additional sutures to close the entire incision. Other wound bonding methods may be used such as dermabond, wound clips, or staples.
- Remove animal from anesthesia system and monitor while consciousness is resumed. Animal should quickly begin ambulating and return to pre-op mobility. Assure that they are able to sufficiently reach food and water. Some moistened food may be placed in the bottom of the cage to assist in recovery. Continue to monitor animal daily for signs of distress or discomfort, and check the incision site for infection, inflammation, and dehiscence. Take appropriate action if any complications to recovery are found.
3. Optional Injection
Note: Once the sponges have been implanted into the animal they can be injected with a solution of cells, drugs, growth factors etc. This allows for analysis of the effects of the injected substance during varied delivery times and administration regimes. If injections are done a control should always be used.
- To inject the sponge with a desired solution, first determine the amount of the substance to administer to the mouse. The substance needs to be concentrated so that the injection volume is less than half the total volume the sponge can hold.
- Prepare a syringe for the injection (for mice we use a 28 G needle).
- Place the animal to receive the injections under anesthesia.
- When the animal no longer responds to the pinch test the injection can be given.
- To give the injection push the needle at a 45° angle thorough the skin at the center of the sponge. Then push the needle into the sponge only going half way through its thickness. The goal is to inject into the exact center of the sponge.
- To check to make sure the needle is in the sponge, slowly lift the needle vertically. If the needle is in the sponge, the sponge should move up with the needle.
- Slowly inject into the sponge and remove the needle. If there are multiple sponges requiring injections it is possible that some of the injected fluid will be pushed out of the previous sites of injection.
4. Sponge Removal
Note: During the sponge removal, handle the sponge with extra caution. Avoid penetrating the sponge with the surgical instruments and prevent excessive bleeding into the sponge by avoiding the major arteries around the surrounding tissue.
- After the animal is euthanized, remove the sponge by holding the skin using a tweezers and making an incision close the sponge.
- Carefully separate the sponges from the surrounding capsule and avoid harvesting extra tissue surrounding the sponge. For assays such as drug delivery or morphometry, less accuracy is required for excision.
5. Representative Results
Removed sponges can be stored under different condition depending on the type of analysis that will be performed. For sectioning, the retrieved sponges can be embedded in a medium for freezing such as Optimal Cutting Temperature (O.C.T.) compound or placed in 10% formalin for embedding and sectioning in paraffin blocks (Figure 3A and B). Best results for sectioning are obtained when the sponge is cut in half across the diameter and embedded cut surface down (Figure 4A and B). Sections of the sponge should be taken so that entire width of the sponge is present. Figure 3A shows two sponges, the left sponge was embedded/sectioned incorrectly and the right sponge was processed correctly.
For analysis, sponges can be placed in an Eppendorf tube and stored at -20 °C until ready for processing. For RNA and quantitative real time PCR analysis sponges should be placed in an Eppendorf tube, flash frozen, and stored at -80 °C. In addition, an RNA preservative, such as RNAlater from QIAGEN, can be used to stabilize the RNA for safe storage at higher temperatures. Wound fluid can be collected by squeezing the sponges over a tube, and pooling together the fluid from multiple sponges to obtain a representative sample. Live cells, such as fibroblast, macrophages and lymphocytes, can also be extracted from the sponges. After removal from the animal, sponges can be place in an appropriate media for the desired type of cells. Sponges can be processed to release the cells by physical (i.e. mincing) or enzymatic (i.e. collagenase) methods.

Figure 1. Dehydrated PVA sponge disks in three different sizes.
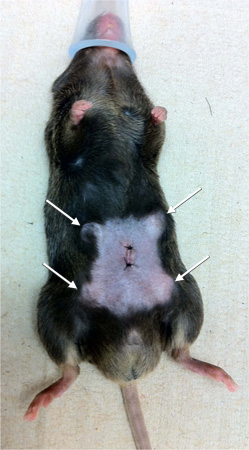
Figure 2. Image of mouse wound-healing model showing position of lateral wound and four PVA sponges.
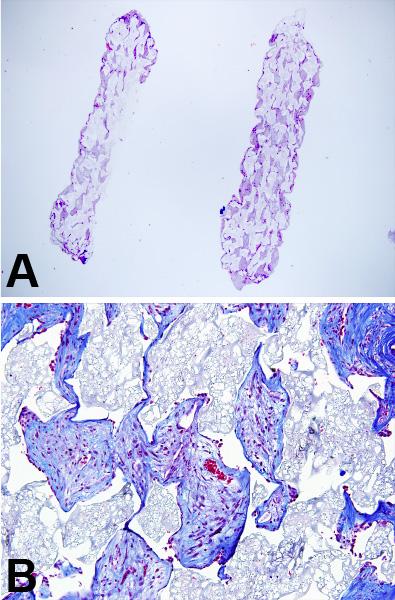
Figure 3. (A) H & E staining at 4x and (B) Trichrome staining at 10x of sectioned paraffin embedded sponges.
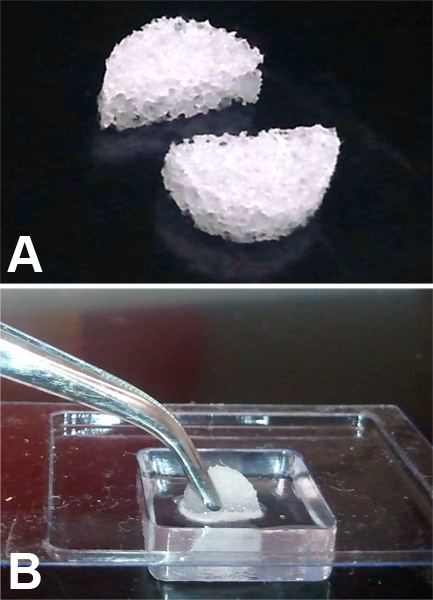
Figure 4. (A) Image of sponge cut in half along the diameter. (B) PVA sponge half, embedded cut side down in OCT.
Discussion
The sponge model of wound healing has multiple variations; it can be performed in many different species including humans and the sponges can also be implanted dorsally 1,2,8. For studies in humans and some other species, sponges may need to be placed in a porous silicone tubing to prevent the sponge from being encapsulated and to allow ease of removal 4,8. The location of the sponge implantation can be altered based on desired results and skill of technician. Dorsal implantation can allow the sponges to move under the skin, while ventral implants are more difficult due to probability of cutting through to the abdominal cavity. The sponges can be set up with a catheter so that drug delivery is constant and controlled, eliminating the need for multiple injections9. Smaller, tubular sponges can even be injected through a needle8. Sponges’ composition is not limited to only polyvinyl alcohol. They can be prepared from many materials exclusively or in combination7.
It is crucial for sponge preparation and placement in the animal to be consistent and properly understood. If sponges are cut from sheets they must be of similar weight for comparability. If the sponge is to be loaded with cells or a treatment, the amount placed in each sponge must be exact. The healing process can vary based on the location of the placement of the sponge in the animal and healing will decrease as distance from the head increases 1,10.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Funding provided by National Institutes of Health (NIH) grant R01-HL088424; Veterans Affairs merit award to PPY.
Materials
| Material Name | Company | Catalogue number | Comments |
| PVA Sponge | Medtronic | CF120 | The size and porosity of the sponge depends on the experiment |
| Scalpel blade size 15 | BD Medial | 371115 | |
| Metzembaum scissors | Thermo Scientific | 79-211 | |
| Hemostat Forceps | Fine Science Tools | 13004-14 | |
| Needle holder | Fine Science Tools | 12004-16 | |
| 5-0 nylon suture | Ethilon | 698 | |
| O.C.T. compound | Tissue-Tek | 4583 |
References
- Efron, D. T., Barbul, A. Subcutaneous sponge models. Methods Mol. Med. 78, 83-93 (2003).
- Gottrup, F., Agren, M. S., Karlsmark, T. Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair. 8, 83-96 (2000).
- Sprugel, K. H., McPherson, J. M., Clowes, A. W., Ross, R. Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am. J. Pathol. 129, 601-613 (1987).
- Alaish, S. M. Comparison of the polyvinyl alcohol sponge and expanded polytetrafluoroethylene subcutaneous implants as models to evaluate wound healing potential in human beings. Wound Repair. 3, 292-298 (1995).
- Davidson, J. M. Animal models for wound repair. Arch. Dermatol. Res. 290, 1-11 (1998).
- Alfaro, M. P. sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J. Biol. Chem. 285, 35645-35653 (2010).
- Andrade, S. P., Ferreira, M. A. The sponge implant model of angiogenesis. Methods Mol. Biol. 467, 295-304 (2009).
- Diegelmann, R. F., Lindblad, W. J., Cohen, I. K. A subcutaneous implant for wound healing studies in humans. J. Surg. Res. 40, 229-237 (1986).
- Efron, D. T., Most, D., Shi, H. P., Tantry, U. S., Barbul, A. A novel method of studying wound healing. J. Surg. Res. 98, 16-20 (2001).
- Lindblad, W. J. Considerations for selecting the correct animal model for dermal wound-healing studies. J. Biomater. Sci. Polym. Ed. 19, 1087-1096 (2008).

