An Ultra-clean Multilayer Apparatus for Collecting Size Fractionated Marine Plankton and Suspended Particles
Summary
Plankton and suspended particles play a major role in the biogeochemical cycles in the ocean. Here, we provide an ultra-clean, low stress method for the collection of various sizes of particles and plankton at sea with the capability of handling large volumes of seawater.
Abstract
The distributions of many trace elements in the ocean are strongly associated with the growth, death, and re-mineralization of marine plankton and those of suspended/sinking particles. Here, we present an all plastic (Polypropylene and Polycarbonate), multi-layer filtration system for collection of suspended particulate matter (SPM) at sea. This ultra-clean sampling device has been designed and developed specifically for trace element studies. Meticulous selection of all non-metallic materials and utilization of an in-line flow-through procedure minimizes any possible metal contamination during sampling. This system has been successfully tested and tweaked for determining trace metals (e.g., Fe, Al, Mn, Cd, Cu, Ni) on particles of varying size in coastal and open ocean waters. Results from the South China Sea at the South East Asia Time-Series (SEATS) station indicate that diurnal variations and spatial distribution of plankton in the euphotic zone can be easily resolved and recognized. Chemical analysis of size-fractionated particles in surface waters of the Taiwan Strait suggests that the larger particles (>153 µm) were mostly biologically derived, while the smaller particles (10 – 63 µm) were mostly composed of inorganic matter. Apart from Cd, the concentrations of metals (Fe, Al, Mn, Cu, Ni) decreased with increasing size.
Introduction
Particles in the ocean play an important role in marine biogeochemical cycles1. Most of the properties of particles, such as size, mineralogy, and composition, can change profoundly from one geological or hydrographical setting to another2. In addition, the distributions of elements in the ocean are also associated with the life cycle of marine phytoplankton: growth, death, sinking, and re-mineralization3,4. Marine particles span at least 4 orders of magnitude in size, ranging from submicron particles to large aggregates (>5 mm). Most particles are biologically derived, from processes such as viral lysis, exudation, secretion, fecal pellet production, etc. Other particles are formed from physical coagulation of cells, cellular debris, or lithogenic materials1. Various chemical and biological characteristics of particles control both the geochemical cycles and biological processes occurring on and within the particles4,5,6. These particles are important habitats as well as food sources for some organisms, such as zooplankton or saprotrophs. Accordingly, the fate of particles is often related to their size, which can be modified by biological processes on and around particles.
Sampling marine particles usually requires filtration, but this approach introduces a certain ambiguity in identifying the properties of particles, since marine particles are not homogenous in composition and size. Suspended particles, mainly composed of small and low density particles that are almost permanently in suspension, are mixed with varying amounts of larger and denser particles in suspension only for a short period of time, depending on hydrodynamic conditions7. The first reports of the trace metal composition of plankton samples were collected by plankton tows or suspending plankton nets on a research vessel8. The authors often found metal particles and paint chips in samples, suggesting a severe problem of contamination during marine particle sampling for chemical analysis. Other efforts include net towing by rubber rafts or using a polyvinyl chloride (PVC)-hand winch3. The difficulty of reliable sampling of particles makes progress in our understanding of the chemical composition of marine particles more difficult, especially for trace elements. As such, most crucial information on the concentration of trace elements in phytoplankton has come from culture studies9,10. This recognition has motivated marine scientists to create new methods for studying particles in the sea over the past thirty years11.
Oceanographers have used various sampling techniques, including shipboard filtration, in situ filtration, and sediment traps11. The processing of large volumes of sea-water to collect non-contaminated samples can be challenging, especially for open ocean and deep waters in which the particle concentrations are very low (0.001 – 0.1 mg/L). It is also necessary to filter large volumes of sea-water to obtain an adequate quantity of particles to measure trace metal concentrations. Some researchers have used the size-fractionation method to separate suspended particles from sinking particles. However, particle size, porosity, density, and shape can all influence particle sinking velocities. Sediment traps are not practical tools to collect suspended particles, since those are designed for sinking particles. Therefore, it is important to develop sampling and treatment methods that can collect sufficient quantities of suspended particles with minimal contamination. Hence, size-fractionation by in situ filtration is still a promising tool in the oceanographer's sampling toolbox, since it can reveal critical information on marine particle dynamics. Here, we describe a successfully tested trace-metal-clean, multi-layer gravity filtration sampling apparatus, which can treat large volumes (120 – 240 L) of seawater on board in one pass from polytetrafluoroethylene (PTFE) coated water sampling bottles in a multi-bottle sampling array. This sampling apparatus uses acid-washed synthetic nylon nets in sequence, and the nets are enclosed within a polycarbonate container to gently collect size-fractionated suspended matter and phytoplankton12,13,14,15 (Figure 1). The aim of this work is to provide a better tool for studying the metal-particle associations and their reaction dynamics in marine environments, and improve our understanding of the fate of a wide variety of planktons, particles, and trace metals in these environments.
Protocol
The following protocol involves working with harmful chemicals. Please read the Safety Data Sheets (SDS) carefully, and follow institutional chemical safety guidelines.
1. Multi-layer Gravity Filtration Sampler Preparation
- Sampler cleaning
- Fill the tubing and filtration unit with 1% (w/v) of anionic protease enzyme detergent solution and soak it for 24 h. Flush the multi-layer gravity filtration sampler with reverse osmosis double distilled water (RO-DDW) thoroughly, then fill it with 0.1% (v/v) hydrochloric acid (HCl, Reagent Grade) and soak for 72 h.
- Thoroughly flush the multi-layer gravity filtration sampler with reverse osmosis double distilled deionized water (RO-DD-DIW) three to five times, at least 20 liters each time, and store the assemblage in plastic bags.
- Particle sample container cleaning/preparation
- Use low density polyethylene (LDPE, 125 mL) or fluorinated ethylene propylene (FEP, 125 mL) bottles as containers for particles. Clean the bottles by soaking them first in alkaline detergent (Micro, 1%), then in 50% (v/v) nitric acid (HNO3, Reagent Grade), then 10% (v/v) HCl solutions for at least 24, 48, and 24 h, respectively. Rinse the bottles with de-ionized water (RO-DD-DIW) between the two soaking steps.
- After a final HCl soaking, rinse the bottles thoroughly with de-ionized water (RO-DD-DIW), and dry the bottles in a clean room or class-100 clean bench.
Attach the cleaned bottle to the multilayer gravity filtration sampler, or seal cleaned bottles in PE zippered bags and double-bag them for transport.
- Assemblage of multi-layer gravity filtration sampler
- Connect six 4 m long chemically resistant thermoplastic elastomer tubes (outside diameter of 0.635 cm) to the six directional inlets on top of the sampler.
- Assemble the three different mesh nylon filters with low-density polyethylene sample containers (125 mL LDPE) in sequence in a clean room (bench) after they are cleaned (see below), with the 10 µm mesh filter positioned on the outside, the 63 µm mesh filter in the middle, and the 153 µm mesh filter on the inside. For transportation, store the multi-layer gravity filtration sampler in two layers of polyethylene (PE) bags, then place it in the polypropylene (PP) shipping container.
2. Sampling
- Sample collection
- Upon arrival at the sampling site, have one person remove the multi-layer gravity filtration sampler from a shipping container on the deck of the research vessel and open the bag with the sampler. Then, have them put on the PE gloves, connect the six 4 m thermoplastic elastomer tubes to the water spigots of six 20 L PTFE-coated sampling bottles on the elevated multi-bottle sampling array, and guide the seawater into this filtration unit. The seawater will flow through the directional inlets, and the particles/plankton will be gently separated/fractionated through the nets and settle into the 125 mL LDPE bottles that are secured at the base of the nets.
- After the seawater has flowed through (usually 120 L for coastal sea water and 240 L for open ocean water), remove each net in sequence (firstly, the 153 µm, then the 63 µm, and finally the 10 µm) in a class-100 clean bench, then spray the net with trace-metal-clean 0.4 µm filtered seawater to rinse out any plankton stuck on the inner surface of the nets. Collect the seawater with concentrated particles/plankton in 125 mL polyethylene bottles.
- Unscrew these bottles from the nets, and filter the solutions with concentrated particles/plankton again through an acid-washed vacuum filtration apparatus with pre-weighed, acid-washed 47 mm, 10 µm pore-size polycarbonate filters under low-vacuum conditions (<5 kPa).
- To collect particles/plankton smaller than 10 µm, wait for at least 20 L of seawater to flow through the sampler, then after that, collect two to five L of water in the 5 L PE container, and filter these sample waters by an acid-washed vacuum filtration apparatus with pre-weighed, acid-washed, 47-mm, 0.4-µm pore size polycarbonate filters.
- After vacuum filtration, rinse the sample filters immediately with high purity DDW water to remove the residue of seawater, minimizing the influence of sea salts on determining the dry weights of particles/plankton. Keep the rinsing volume to only a couple of milliliters to prevent damaging the fragile plankton.
- Then, after this rinsing step, carefully remove the filter from the vacuum filtration unit, store the sample filters in acid-washed, pre-weighed acrylic plastic petri dishes, and seal in resealable plastic bags. Keep the bags in a -20 ˚C freezer onboard until returning back to a land-based laboratory for further sample pretreatment and chemical analysis.
3. Sample Treatment
- Freeze drying and digestion of particles
- Place the filters with particle samples in the collector chamber of the freeze-drying machine, and turn on the machine. As the machine temperature reaches -40 °C, turn on the vacuum pump of the machine and start the freeze drying processes.
NOTE: The vacuum level should be maintained steadily below 0.12 mBar. Please read the user manual carefully and follow the manufacturer's guidelines for each step. - After 72 h, turn off the freeze-drying machine, remove the dried filters and weigh them. Then, place dried sample filters into pre-weighed perfluoroalkoxy alkane (PFA) vessels (60 mL capacity), and add 3 mL of concentrated ultrapure nitric acid into the vessels2,3,6,7.
- Tighten the vessels with a torque wrench to a constant torque of 2.5 kg-m, and place the vessels in a conventional oven at 130 °C for 12 h for the first digestion sequence. After cooling, remove the vessels from the oven, open the vessels, and add 2 mL of ultrapure hydrofluoric acid into the vessels2,3,6,7.
- Tighten the vessels with a torque of 2.5 kg-m, and place the vessels in a conventional oven at 130 °C for 12 h, which is the second digestion sequence. After cooling, open the vessels and add 16 mL of 4.5% ultra-pure boric acid solution into the vessels2,3,6,7.
- Tighten the vessels to a constant torque of 2.5 kg-meters, and digest the samples in oven at 130 °C for 12 h for the final digestion sequence. After cooling, weigh each vessel and determine the final mass and specific mass of each digested solution to yield a final digestant volume.
NOTE: Specific mass is determined by measuring the weight of exactly 1.00 mL of digestant. - Carefully pour the digestant into 30 mL acid cleaned PE bottle for further trace metal analysis.
- Place the filters with particle samples in the collector chamber of the freeze-drying machine, and turn on the machine. As the machine temperature reaches -40 °C, turn on the vacuum pump of the machine and start the freeze drying processes.
- Trace Metal Analysis
- Determine trace metal concentrations (Cd, Cu, Fe, Mn, Ni, and Al) in digested solutions of particles using a graphite furnace atomic absorption spectrometer (GF-AAS)6.
- As an accuracy test, use certified reference material (CRM), such as marine sediment reference materials from the National Research Council of Canada, estuarine sediment standard reference material from the National Institute of Standards and Technology of the USA, and plankton reference material from the European Commission's science and knowledge service. The process gives 95% to 107% recovery of the certified value for the trace metals provided in the CRM.
Representative Results
With the development of modern oceanography, it is now a common practice to use "clean techniques" to obtain accurate trace metal concentrations in marine particles or plankton. Since most particles in natural waters are in the low mg/L to µg/L range, the treatment of large volumes of seawater is necessary to investigate geochemical and biological effects of trace metals on various particles in ambient environments. With the use of clean, multi-layer gravity filtration ("CATNET") sampling techniques (Figure 1), good agreement was found between particle concentrations determined using conventional pressurized dead-end filtration and those collected by CATNET, using a data set of coastal seawater sampled from the west coast off Taiwan (Figure 2). More than 90% of those particles were small (0.4 – 10 µm). When compared ambient unfiltered seawater to CATNET filtered seawater (<10 µm), using this protocol produced very low blanks and no noticeable contamination (Table 1). For particles collected at the depth of the chlorophyll-a maximum in the South China Sea between 3/26/2002 and 3/28/2002, most particles (>80%) resided in smaller (0.4 – 10 µm) particles. Larger particles, i.e., zooplankton (>153 µm), clearly showed diurnal vertical migration patterns, while the concentrations of smaller particles remained almost unchanged (Figure 3). The live zooplankton observed in the sampling bottles indicated the gentleness of the filtration process10. In the surface water of the Taiwan Strait, the analytical wet chemistry and sampling techniques described here are used to measure marine particle distributions and compositions. The histogram of the average metal concentrations in the suspended particles (µg/g) among the different size fractions collected varied dramatically, spanning over five orders of magnitude. The concentrations varied significantly in different particle size groups: 0.4 – 10 µm, 10 – 63 µm, 63 – 153 µm, and >153 µm. Generally, most particles were enriched in Fe and Al, and the concentrations decreased with increasing size, except for Cd, which increased with increasing size, possibly caused by a bio-concentration process3,10,14 (Figure 4).
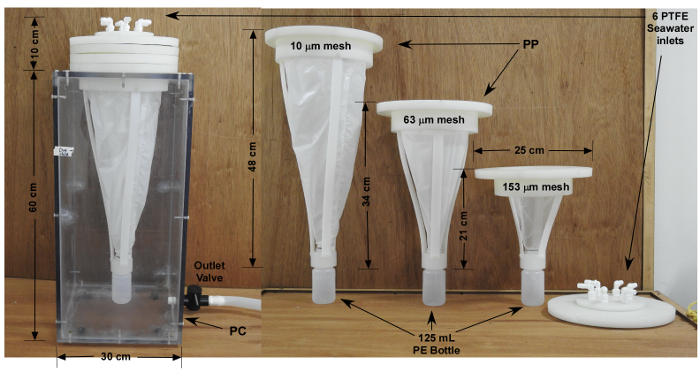
Figure 1: Ultra-clean multilayer gravity filtration sampler for collecting size fractionated marine plankton and suspended particles (CATNET). This particle collection sampler is made of polycarbonate and polypropylene materials, and is fitted, in sequence, with 153 µm, 63 µm, and 10 µm changeable nylon nets. Water samples are drawn from six 20-liter PTFE-coated sampling bottles on the elevated multi-bottle sampling array, connected to the inflow ends of the size-fractionated filtration apparatus via acid-washed thermoplastic elastomer tubes. This filtration system effectively prevents possible contamination while collecting the samples onboard, and particles are gently separated in different sizes sequentially through the nets, sinking into the LDPE bottles at the bottom of each net. The "CATNET" was nicknamed by the co-author Miss Wen-Huei Lee for a short abbreviation of "Dr. Cat's ultra-clean multi-layer collection net", so that users could distinguish the apparatus and filtration method with regards to the designer/inventor, Dr. Liang-Saw "CAT" Wen. This device was patented until May 9th, 201512. Please click here to view a larger version of this figure.
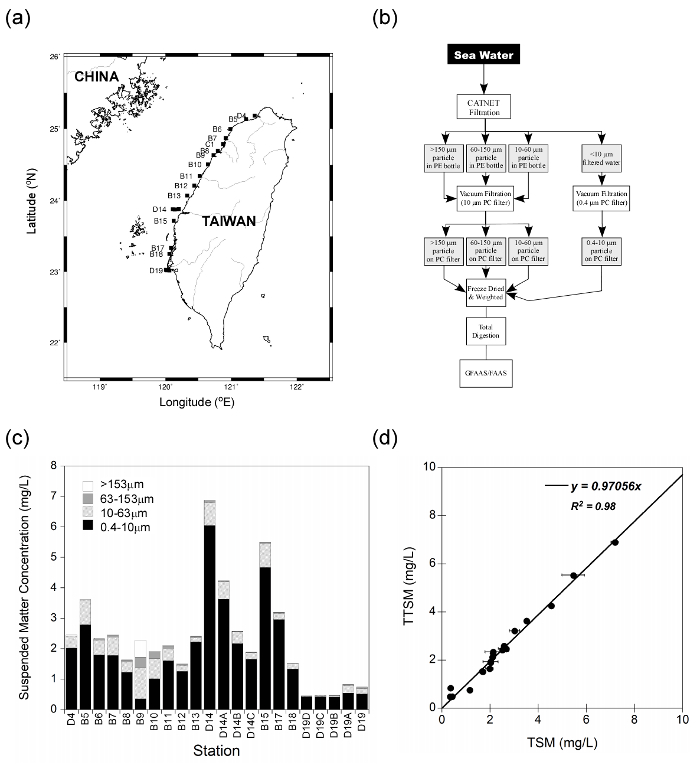
Figure 2: Comparison of total suspended materials (TSM) collected from coastal waters by two independent filtration methods. Coastal water samples at a depth of 5 meters were collected on board at the R/V Ocean Research II in April 2007 (OR2-1432, 2007/4/21-4/23). (a) Sampling stations, (b) Schematic of sampling procedure, (c) Different size particle concentrations of each sample sites determined by CATNET method, and (d) Comparison of particle concentration determined by conventional filtration method (TSM) and CATNET method (TTSM). The error bars are the standard deviations of duplicated samples as measured by the TSM. Very good agreement was found between suspended particle concentrations determined using the two independent methods in separate aliquots of the same samples. There were 22 sampling sites, and two samples for each site were collected and filtered directly by the commonly used, pressurized dead-end filtration device7,11,16 (total suspended materials, "TSM", particle weight of larger than 0.4 µm), and another sample was collected by CATNET followed by low-pressure vacuum filtration (total amount of suspended materials, "TTSM", the sum of weights of 0.4 – 10, 10 – 63, 63 – 153, and >153 µm particles; only done once due to operation time). A large concentration range implies that the techniques are suitable for particle studies in distinct environments where concentrations show significant differences. Please click here to view a larger version of this figure.
| Analytes | Field Double-Deionized Water Blanks | Ambient Seawater (<0.4 µm) | CATNET Filtrate Seawater (<10 µm) |
| Nitrite (µM) | n.d. | 0.23 | 0.22 |
| Nitrate (µM) | n.d. | 1.4 | 1.45 |
| Ammonium (µM) | n.d. | 0.081 | 0.088 |
| Phosphate (µM) | n.d. | 0.16 | 0.15 |
| Silicate (µM) | n.d. | 4.01 | 4.05 |
| DOC (µM) | n.d. | 83 | 81 |
| Cu (nM) | 0.08 | 0.91 | 0.85 |
| Fe (nM) | 0.005 | 0.34 | 0.35 |
| Ni (nM) | 0.01 | 2.45 | 2.35 |
Table 1: Nutrient and trace metal concentrations in procedure blank waters, ambient seawater, and CATNET filtered waters. Illustration of nutrient and trace metal concentrations in 3 field blanks (high purity water treated as samples in the field), and ambient water (115˚34'E, 18˚15'N; 80 meter depth) before and after CATNET filtration, which indicate the effectiveness of the described protocol. There was no evidence for increased concentrations due to confinement stress effects (unnatural excretion because of colliding, unnatural light exposure, temperature shock, vigorous mixing, cell rupture, etc.) or contamination (trace metals in washes and collection bottles, collection gear, fittings and wires, plastic closures, etc.). Low field blanks were also achieved. Not detectable: n.d.
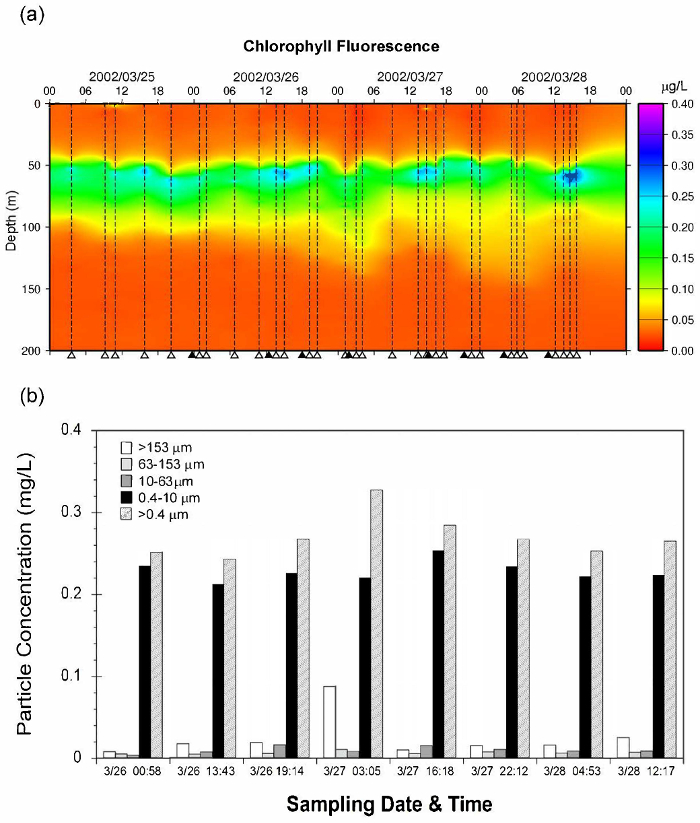
Figure 3: Temporal variations of (a) Chlorophyll fluorescence in the euphotic zone, and (b) different size particles collected at depths of Chlorophyll-a maximum. Samples were collected on board at the R/V Ocean Research I in March 2002 (OR1-639, 2002/3/21-3/30)13. The dashed lines with triangles in (a) indicate the CTD downcast and hydrographic data retrieval times; the solid triangles denote the sampling time for particles at depths of chlorophyll maximum for CATNET deployments. While some concentrations of smaller particles remained almost unchanged, the zooplankton (>153 µm) clearly showed nighttime vertical migration patterns. Please click here to view a larger version of this figure.
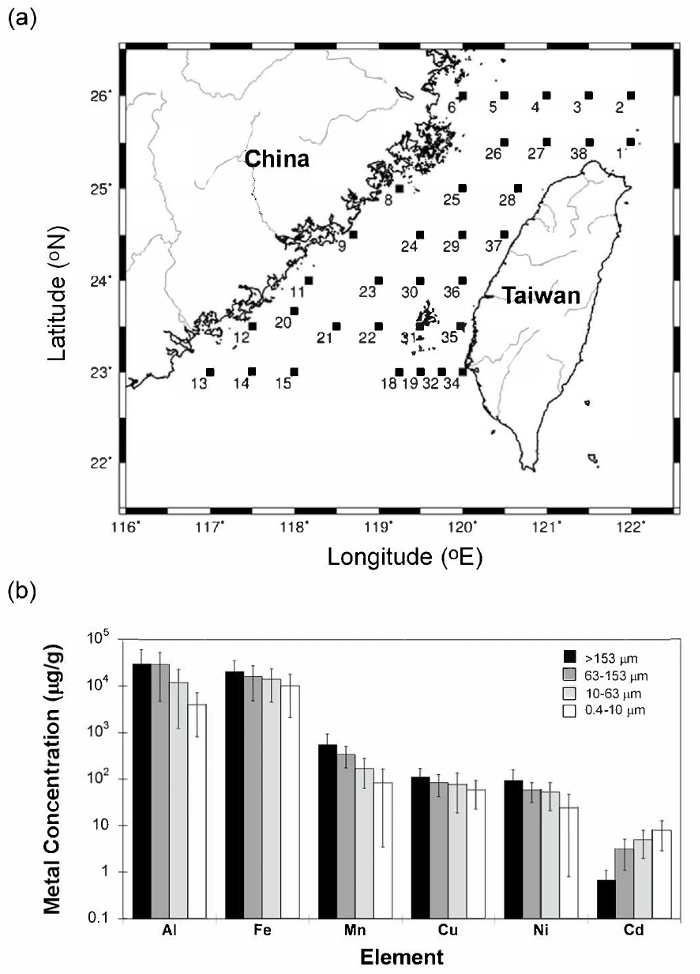
Figure 4: Comparison of the average metal concentrations in the dried suspended particles (µg/g) among the different sizes. (a) Samples were collected on board at the R/V Ocean Research II during the summer of 2007 (OR2-1444, 2007/5/31-6/6). (b) Average metal concentrations with standard deviations of the dried suspended particles (µg/g) for all 35 samples among the different sizes. Overall, the trace-metal compositions varied dramatically, spanning over five orders of magnitude. The trace metal concentrations also varied significantly in the differently sized particles collected (0.4 – 10, 10 – 63, 63 – 153, and >153 µm) in surface waters of Taiwan Strait; generally, the concentrations decreased with increasing size, except for Cd. Please click here to view a larger version of this figure.
Discussion
Obtaining reliable trace metal concentrations on plankton and suspended particles in natural waters, which are generally present at very low concentrations, requires great care during sample collection, processing, pretreatments, and analysis, with the aim of reducing contamination. Therefore, the procedures to design and prepare sampling gear, sample containers, and materials used to collect and process samples are all critical steps toward obtaining high-quality data for trace metals in marine environments. With the advance of new particle-collecting methods in recent decades, our knowledge of particle dynamics as well as trace element biogeochemistry is also broadening. In this paper, we have illustrated a sequential size-fractionating technique that can be used to study the distribution and composition of marine plankton/particles. In the seawaters we investigated, trace-metal compositions varied dramatically in particles with various sizes and origins, spanning over five orders of magnitude. Generally, most small particles (0.4 – 10 µm) were enriched with trace metals such as Fe and Al, and the concentrations decreased with increasing size3,10,14. Compared with conventional dead-end filtration, the results of total particle concentrations in coastal seawaters indicated that using the outlined protocol yielded good agreement.
The protocol described here can be easily applied to collection in different types of marine environments, estuarine and coastal waters, lakes, or open oceans. Sample volume can be adjusted if greater or lesser amounts of particles are required. In highly turbid waters, samples should still be collected cleanly, and great care has to be taken to remove any residual particles adhering to the Nylon net before processing the next water sample. The cleaning and preconditioning steps, and the awareness of "trace-metal sampling clean techniques" are critical for satisfactory mass balance and consistently good results. This work demonstrates that the determination of trace metal distribution in marine plankton and suspended particles requires "clean techniques" that include sampling and separation, and this device and related processing gives improved results.
The range of large volume seawater for which this protocol is applicable implies that investigations of particle distributions and behavior can also be conducted effectively in various marine environments. Collection of particles in discrete samples followed by chemical characterization still has spatial and temporal limitations, which potentially introduce bias into the interpretations due to a potentially incomplete account of the particle field. However, by comparing results of various particle collection methods, we can further expand the scope of particles/plankton research by providing details of the reactions and processes governing different sizes of particles, and determining their corresponding biogeochemical dynamics. The continuing research on particles/plankton will shed light on their roles in the ocean.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Miss Pi-Fen Lin, Mr. Wei-Lung Tseng, Miss Pei-Hsuan Lin, and Dr. Jia-Lu Chuan for their assistance during the field sampling and lab analysis for the practical development and application of "CATNET." The assistance of crew and technician on board research vessel Ocean Research-I and Ocean Research-II during the sampling expeditions is greatly appreciated. This work was supported partly by Taiwan Ministry of Science and Technology of grants 91-2611-M-002-007, 95-2611-M-002 -009, 96-2611-M-002-004, 97-3114-M-002-006, 104-2611-M-002-019. This manuscript is written in memory of Miss Wen-Huei Lee for her immense dedication and contribution to marine researches in Taiwan.
Materials
| thermoplastic elastomer (C-Flex) Tubings | Cole Palmer | EW-06424-67 | O.D. 0.635 cm, Opaque White 1/8"ID x 1/4"OD, 25 ft/pack |
| LDPE Bottle (Nalgene) | ThermoFisher Scientific | 2103-0004 | 125 mL, Nalgene Wide-Mouth LDPE Bottles with Closure |
| anionic protease enzyme detergent detergent (Tergazyme) | Alconox | 1104-1 | 1×4 lb box (1.8 kg) |
| Hydrochloric Acid | Sigma-Aldrich | 258148 | Reagent grade |
| Nitric acid | Sigma-Aldrich | 695025 | Reagent grade |
| alkaline detergnet (Micro) | Cole Palmer | EW-99999-14 | Micro-90 Cleaning Solution |
| polycarbonate filter, 47 mm, 0.4 µm | Sigma-Aldrich | WHA111107 | Whatman Nuclepore Track-Etched Membranes, diam. 47 mm, pore size 0.4 μm, polycarbonate |
| polycarbonate filter, 47 mm, 10 µm | Sigma-Aldrich | WHA111115 | Whatman Nuclepore Track-Etched Membranes, diam. 47 mm, pore size 10 μm, polycarbonate |
| PFA vessel, 60 ml capacity | Savillex | 300-060-03 | 60 mL Digestion Vessel, Flat Interior, Flat Exterior, Buttress Threaded Top |
| Nitric acid, ultrapure | Seastar Chemicals | N/A | BASELINE Nitric Acid |
| HF, ultrapure | Seastar Chemicals | N/A | BASELINE Hydrofluoric Acid |
| Boric acid, ultrapure | Seastar Chemicals | N/A | BASELINE Hydrobromic Acid |
| polyethylene (PE) gloves | Safty Zone | GDPL-MD-5 | Clear Powder Free Polyethylene Gloves |
| Multiple layer filtering and collecting device | Sino Instrumnets Co. Ltd | not available | Multiple layer filtering and collecting device, CATNET |
| 10 um Nylon filters, Nitex | Dynamic Aqua-Supply Ltd. | NTX 10 | Nitex – Standard Widths (40 – 44 inches) |
| 60 um Nylon filters, Nitex | Dynamic Aqua-Supply Ltd. | NTX 60 | Nitex – Standard Widths (40 – 44 inches) |
| 150 um Nylon filters, Nitex | Dynamic Aqua-Supply Ltd. | NTX 150 | Nitex – Standard Widths (40 – 44 inches) |
| torque wrench | Halfords | 200238 | Halfords Professional Torque Wrench 8-60Nm |
| multi-bottle sampling array, Rosette | General Oceanics | Model 1018 | Rosette Sampler |
| PTFE-coated sampling bottles, GO-Flo | General Oceanics | 108020T | GO-Flo water sampler teflon coated |
| Marine sediment reference materials | National Research Council Canada | MESS-3 | |
| Estuarine sediment standard reference material | National Institute of Standards and Technology | 1646a | |
| Plankton reference material | The European Commission's science and knowledge service | CRM414 |
References
- Jeandel, C., et al. What did we learn about ocean particle dynamics in the GEOSECS-JGOFS era. Progr. Oceanogr. 133, 6-16 (2015).
- Lam, P., et al. Methods for analyzing the concentration and speciation of major and trace elements in marine particles. Progr. Oceanogr. 133, 32-42 (2015).
- Collier, R., Edmond, J. The trace element geochemistry of marine biogenic particulate matter. Progr. Oceanogr. 13, 113-199 (1984).
- Donat, J. R., Bruland, K. W., Steinnes, E., Salbu, B. Trace elements in the oceans. Trace Elements in Natural Waters. , 247-280 (1995).
- Wen, L. -. S., Santschi, P., Tang, D. Interaction between radioactively labeled colloids and natural particles: evidence for colloidal pumping. Geochim. Cosmochim. Ac. 61, 2867-2878 (1997).
- Wen, L. -. S., Warnken, K., Santschi, P. The role of organic carbon, iron, and aluminium oxyhydroxides as trace metal carriers: Comparison between the Trinity River and the Trinity River Estuary (Galveston Bay, Texas). Mar. Chem. 112, 20-37 (2008).
- Hurd, D., Spencer, D. Marine particles: analysis and characterization. American Geophysical Union. , (1991).
- Martin, J. H., Knauer, G. A. The elemental composition of plankton. Geochim. Cosmochim. Ac. 37, 1639-1653 (1973).
- Morel, F., Price, N. M. The biogeochemical cycles of trace metals in the oceans. Science. 300, 944-947 (2003).
- Ho, T. -. Y., et al. The elemental composition of some marine phytoplankton. J. Phycol. 39, 1145-1159 (2003).
- McDonnell, A., et al. The oceanographic toolbox for the collection of sinking and suspended marine particles. Prog. Oceanogr. 133, 17-31 (2015).
- Wen, L. -. S., Li, W. -. H., Zhuang, G. -. Z. . Multiple layer filtering and collecting device. , (2005).
- Ho, T. -. Y., Wen, L. -. S., You, C. -. F., Lee, D. -. C. The trace-metal composition of size fractionated plankton in the South China Sea: biotic versus abiotic sources. Limnol. Oceanogr. 52, 1776-1788 (2007).
- Hsu, R., Liu, J. In-situ estimations of the density and porosity of flocs of varying sizes in a submarine canyon. Mar. Geol. 276, 105-109 (2010).
- Liao, W. -. H., Yang, S. -. C., Ho, T. -. Y. Trace metal composition of size-fractionated plankton in the Western Philippine Sea: the impact of anthropogenic aerosol deposition. Limnol Oceanogr. , (2017).
- Grasshoff, K., Kremling, K., Ehrhardt, M. . Methods of seawater analysis. , (2007).

