Home-Based Transcranial Direct Current Stimulation Device Development: An Updated Protocol Used at Home in Healthy Subjects and Fibromyalgia Patients
Summary
This study provides an updated home-based tDCS protocol that enables subjects to receive the beneficial effects of tDCS at home with an easy to use device with settings to control the use and dosage, enhancing the feasibility for long-term use at home.
Abstract
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation (NIBS) method, which modulates the membrane potential of neurons in the cerebral cortex by a low-intensity direct current. tDCS is a low-cost technique with minimal adverse effects and easy application. This neurostimulation method has a promising future to improve pain therapy, treatment of neuropsychiatric disorders, and physical rehabilitation.
Current studies demonstrate the benefits of using tDCS over consecutive multiple sessions. However, the daily displacement to the specialized centers, travel costs, and disruptions to daily activities are some of the difficulties faced by patients. Thus, to be more comfortable, easy-to-use, and not disrupt daily commitments, a home-based tDCS was designed. Therefore, the objective of this study was to evaluate the feasibility of a portable tDCS device for home use in healthy subjects and fibromyalgia patients.
Despite increased tDCS use and a reasonably large body of research on the effects across a range of clinical conditions, there is a limited amount of research on developing secure devices that guarantee the dose and contain a block system to avoid excessive use. Therefore, we used a tDCS device with a security system to permit daily use for 20 minutes with a minimal interval of 12 hours between sessions. A programmer preconfigures the equipment, which has a neoprene cap that allows the electrode positions in any assembly, according to individualized protocols for treatments or research. After, researchers can assess the effectiveness of treatment, and its adherence using information kept in the device software.
Results suggest that the device is feasible for home use, with proper monitoring of adherence and contact impedance. There were reports of a few adverse effects, which do not differ from those reported in the literature in studies with the treatment under direct supervision.
Introduction
Non-invasive brain stimulation (NIBS), such as transcranial magnetic stimulation (TMS) and tDCS, is emerging as a therapeutic option for various conditions, such as neuropsychiatric disorders1,2, pain syndromes3,4,5,6,7, and rehabilitation8,9. tDCS is a form of neurostimulation that modulates the membrane potential of neurons in the cerebral cortex by a low-intensity direct current (1-2 mA). The electrical current is applied directly to the scalp at the targeted brain area (i.e., primary motor cortex, prefrontal dorsolateral, etc.) through sponge electrodes soaked with saline solution or rubber electrodes with conductive gel10. It is a low-cost technique, with easy application and practically no adverse effects.
However, the use of long-term tDCS becomes impracticable for many patients with chronic disease. When we analyze the current evidence about tDCS, we can observe that most protocols used fewer than 20 sessions and also, the treatment was administered in a specialized center6. Nonetheless, it is a frequent complaint of patients that they spend time and money to go the center.
In the real world, there are several everyday difficulties of patients receiving this kind of therapy because frequently they are suffering from refractory pain and/or they have severe psychiatric or neurological disorders. Many of them have difficulties walking or are wheelchair users. An ideal device should guarantee consecutive sessions for long-term therapies as well as be able to run more extensive clinical trials with larger sample sizes. Furthermore, the device should have a block system to guarantee the dose of stimulation while avoiding any abusive or excessive use. Also, the development of a device to be used without direct supervision can test its effectiveness in different samples, either as a unique method or combined with pharmacological treatments, cognitive tasks or physical functions11,12. Thus, we need to take into account that the repeated sessions are an intrinsic characteristic of this kind of treatment according to a vast set of evidence. A clinical response usually requires repeated sessions of stimulation during extended periods5,13,14. In this context, there exists a gap to develop a portable tDCS device for home use, at the lowest cost, that is easy to handle and guarantees the safety and benefits observed in studies under direct supervision at care centers.
Presently the most advanced system available to use tDCS at home requires remote assistance, which is a considerable barrier to large scale treatment, especially in the long-term. In fact, in real life, it is not feasible to maintain a long-term intervention if the patient and the operator controlling the device remotely need to be connected to a video conferencing platform simultaneously. The remote support is a barrier to maintain the treatment because both the patients and the operator controlling the remote need to be available at a pre-determined time of day for the treatment to occur. This is not practical, and it withdraws the patient's autonomy to decide what is the best moment for the treatment session according to their daily life routine15,16. Taking this into account, a tDCS device to be used at home should be practical and friendly for users. The tDCS device presented in this study is simple and with a short training can give the ability for the participants' to use the apparatus at home.
An essential characteristic of our device is that it was designed for home use. For this reason, we include a block system to avoid any indiscriminate use. Thus, an untrained individual can use the device, and the technique can be reproduced across studies to make comparison of results possible between clinical trials11,15,17. Aiming to reduce difficulties related to long-term treatment, we developed a protocol using a home-based tDCS device; here we present a protocol for a safe and straightforward portable tDCS device, preventing its improper use16. We validated this protocol of the home-based tDCS in both healthy subjects and fibromyalgia patients, as well as showed its feasibility as attested by electrophysiological parameters (i.e., motor evoked potential), and clinical measures.
We included healthy subjects and fibromyalgia patients in this study. Fibromyalgia is a syndrome that comprises chronic widespread musculoskeletal pain accompanied by other symptoms such as depressive symptoms, fatigue, sleep disturbance, and morning stiffness. Many patients experience continued symptoms despite initial pharmacological treatment at the maximum tolerated dose. Hence, we advise studying different treatment modalities, including non-pharmacological measures such as the NIBS techniques, in this context, the home-based tDCS. tDCS is a potentially viable option in the treatment of chronic pain, as demonstrated in several studies4,6,7. The development of a home-based tDCS allows the creation of long-term clinical trials, and a better understanding of the cumulative effect of tDCS for chronic disorders (i.e., chronic pain, depression, rehabilitation after stroke, etc.).
Despite the fact that tDCS use has increased and there is a reasonably large body of research on its effects and impact across a range of clinical conditions, a surprisingly limited amount of research has been conducted on developing a device that can be feasibly and securely used at home. Therefore, the objective of this study was to evaluate the feasibility of the tDCS device for home use (monitoring of adherence and contact impedance) in healthy subjects and fibromyalgia patients.
Protocol
This study was approved by the institutional ethics committee at the HCPA and obtained written informed consent from all subjects. Details of the study with a sample of healthy subjects and fibromyalgia patients are in Figure 1.
1. Participant Recruitment and Screening
- Recruitment
- Perform a screening questionnaire over the telephone to assess eligibility and request confidential medical information (psychiatric history, regular tobacco use, drug abuse and alcohol use as exclusion criteria).
- Conduct a baseline screening to assess the participants' aptitude of home-based tDCS.
- Use the following Inclusion/Exclusion criteria.
- For Healthy Subjects (HS): include healthy male and female volunteers who have completed higher education, are right-handed and aged between 18 to 40 years.
- For Fibromyalgia (FM) patients: include patients with diagnosis of fibromyalgia based on American College of Rheumatology criteria, literate, female and aged between 18 to 60 years.
- Exclude contraindication to tDCS use: history of brain surgery, tumor, stroke, implantation of intracranial metal, pregnant or breastfeeding.
- Exclude subjects who receive less than 80% of the stipulated sessions.
2. Proposed Equipment
Note: The Biomedical Engineering department at the Hospital de Clínicas de Porto Alegre in partnership with the Laboratory of Pain & Neuromodulation developed the home-based tDCS. To solve the difficulty of correct placement of the electrodes without the help of another person, we created a tDCS device and a cap that allows the use of two electrodes in several assemblies.
- tDCS Device
NOTE: The device has dimensions of 110 mm x 75 mm and a weight of 165 g. Consumption while in use is 20 mA and 10 µA on stand-by and can only be programmed by researchers.- Set the device parameters: the fixed number of stimulation sessions, the minimum interval between two consecutive sessions, the intensity of the electric current, duration of stimulation, the rise and descent time of the current, and stimulation mode: active or sham.
- Cap/Silicone Probes/Electrodes
NOTE: The cap's material is neoprene, which facilitates contact of the sponges to the scalp, keeping the sponges fixed at the stimulation site. The cap has a Velcro strap attached to the chin ensuring the cap does not move during the stimulation.- Choose the correct dimensions of the cap. The cap allows facilitated contact of the sponges to the scalp, keeping the sponges fixed at the stimulation site, and has three sizes: small (38 cm x 55 cm), medium (39 cm x 57.5 cm) and large (40 cm x 59 cm).
- Put the cap on the participant and measure/mark the chosen region.
- Remove the participant's cap and drill the cap at the desired location. Attach the electrodes and connect the silicone cannulas and filled syringes of the saline solution. Form electrodes from 35 cm2 conductive rubber electrodes coated with a vegetable sponge and a metal piece that connects it to the silicone cannulas (Figure 2).
- Protocols
- Define a protocol for participants. For healthy subjects, use anodal stimulation over the left primary motor cortex (M1) and cathodal stimulation over the contra-lateral supra-orbital area (Figure 3A), 2 mA for 20 min, in a total of 10 consecutive sessions.
- Define a protocol for patients. For fibromyalgia patients, use anodal stimulation over the left dorsolateral prefrontal cortex (DLPFC) (Figure 3B), 2 mA for 30 min, in five consecutive days (Monday to Friday) in a total of 60 sessions. Randomize FM patients to one of two treatments (active or sham tDCS). A researcher not involved in data collection performed the randomization and used the computer program entitled Randomization.com.
NOTE: During active stimulation, there is ramp-up time of 20 s for the current to go from zero to 2 mA and a ramp-down time that also takes 20 s for the current to go from 2 mA to zero to end the stimulation. In this way, the participant does not suffer from an abrupt interruption of the current and the visualization of spots or light spots, known as phosphenes, produced from electrical or magnetic stimulation19,20. The sham application has the same ramp-up and ramp-down time, but in three moments: at the beginning, in the middle (at 15 min) and at the end of the session (at 30 min), to convince the participant that they are receiving the stimulation.
3. Baseline Protocol Visit – tDCS Training
- Prepare the materials for each participant: a case containing a neoprene cap, a tDCS device, a battery charger, 2 electrodes, 2 silicone cannulas attached to the electrodes, 20 syringes with 10 mL of saline and two syringes of additional physiological saline solution, and one bottle of additional physiological saline solution.
- Hold a training session with the participant and inform them about the details of the device and the step-by-step process for self-stimulation administration. Participants should also watch an instructional video about the use of tDCS at home.
- Training Session
- Inspect the skin for any injury or something that contraindicates the tDCS use.
- Place the participant in front of a mirror and separate their hair in order to reveal the area to be stimulated, according to the protocol of the study.
- Clean the surface of the skin to remove creams, dirt or grease.
- Put the cap on the head with the front seam between the eyebrows. The cap is to be neither tight nor loose but comfortable.
- Connect the electrode cable to the device and turn on the equipment.
- Sequence of screens
- Turn on the device and the first screen will appear: Opening screen (Figure 4A).
- Wait a few seconds and the second screen will appear: Battery status (Figure 4B). In the sequence, the third screen will show the date and time (Figure 4C).
- As the next screen is the command wear the cap, push the central button after making sure the cap is well secured (Figure 4D).
- Wait for the command inject saline. Make sure the saline is fed slowly to the electrodes using the already connected syringe, using 6 mL for each electrode. Press the central button after injecting the saline (Figure 4E).
- The sixth screen is the command to start the session. The participant must press the center button when ready to begin the stimulation (Figure 4F).
- As the seventh screen shows the beginning of the stimulation, see in the display: session duration, battery charge, and two lines. The upper line shows the intensity (I), and the bottom line shows the resistance (R) (Figure 4G).
NOTE: The intensity will gradually increase until reaching 2 mA (or another defined intensity). The resistance should approximately reach the middle of this line (5 kΩ), and the investigator can configure the device to the maximum impedance limit they want. - Wait for the end of the stimulation, and after twenty minutes (or other stipulated time), the tDCS device powers off and stimulation ceases, saving the session data to the device software (Figure 5).
NOTE: The device records each session, with the day and time of stimulation, resistance and total duration of stimulation.
- Safety features
- Define tolerability. After beginning the stimulation, ask the participant about comfort to the stimulus. If the participant reports discomfort, wet the sponges with saline solution to decrease these effects or reduce current intensity to 1.0 mA (or another defined intensity).
- Watch for high resistance. If resistance is too high (Figure 6A), the device will issue an alarm warning: "adjust the cap and inject extra saline" (Figure 6B). At this point, adjust the electrodes on the head and inject some more saline, trying not to exceed 10 mL. High impedance will occur when there is poor contact, a low amount of saline or when the electrical current changes by more than 10%.
- Stop the session if necessary. The central button will need to be pressed for a few seconds, followed by pressing the switch for the off button after which a message "Shut down?" will appear (Figure 7).
- Do not repeat session in 24 h. If the stimulation session lasted less than 50% of the total time, it is possible to repeat the session on the same day. However, if the session lasted more than 50% of the whole time, it isn't possible to replicate the stimulation on the same day, as the initial session will be considered a valid one.
- Charge the battery. The device contains a 9-volt battery, which is rechargeable. When the battery is at 50%, it is necessary to charge it. For this, it is required to disconnect the electrode cable and swap it with the charger cable, because it is not possible to charge the battery during a stimulation session.
4. Home-based tDCS Sessions
- Instruct the participant to choose a quiet moment in his or her day for the session, to not be interrupted and to avoid having to stop the procedure. The activity of the participant should be oriented according to the study protocol and should be the same for all sessions.
- Adverse effects
- Instruct the participant to correctly fill the adverse effects diary immediately after the tDCS home session. Patients with FM also were instructed to fill the pre (before the session) and post-estimate pain levels, immediately after the session.
- Team availability
- Give participant all the information in written form and a contact phone number. This number must be available 24 h a day because if the participants had doubts or some problem in using the device, they should be able to contact a team member at any time.
- Ask the participant the approximate time of day the session will take place. Thus, a team member should be aware of possible demands.
NOTE: After the end of the protocol, the participant should do the final evaluation and give back the tDCS device and adverse effects diary.
- Access software information
- Access the recorded information at the end of the treatment. To do this, use a computer and a cable to extract the information saved in the software. This data shows all the sessions performed and presents the values of the current intensity used and the contact impedance of each session. The participant does not have access to this information (Figure 8).
Representative Results
A total of n=20 healthy subjects were recruited (8 men / 12 women) between March 2015 and April 2016. One participant was excluded, due to having performed only 50% of the sessions. Thus, a total of 19 subjects completed the experiment. The mean age was 26.31 years (±4.89).
A total of n=8 fibromyalgia patients were recruited between November 2016 and April 2017 and randomized to two groups: FM active and FM sham. The mean age was 49.5 years (±8.48). Patients who experienced the sham condition received a questionnaire of "blinding validation" asking what stimulation they believe they received. All of the patients answered that they had received active stimulation.
We measured adherence by counting the number of completed sessions, as verified by the records on the software. In the study with HS, there was a 90% adherence to the proposed sessions, totaling 19 training sessions and 171 valid sessions (total: 190). In the study with FM patients, there was a 94% adherence to the proposed stimulation sessions, totaling eight training sessions and 227 valid sessions for FM active and 225 valid sessions for FM sham (total: 460). The total number of valid sessions in the healthy and fibromyalgia sample was 650 sessions, with overall adherence of 93%.
The contact impedance of the sample of HS had a mean of 2.93 (±1.04) and the study with FM patients (only active tDCS) was 2.82 (±1.15). The mean of contact impedance taking into consideration both samples (n = 416) was 2.87 (±1.13) (Figure 9).
The adverse effects were similar between the HS and FM active groups (although the FM group had some items more frequently than the HS group). The sham group had adverse effects, but these were smaller than those found in the other groups (Figure 10).
A total of n=47 sessions were not performed by the participants (HS=19 and FM=28). The justifications were: prescheduled social activities, forgetfulness, and technical problems in the cable and battery. Three participants needed to replace the battery, which did not recharge properly. None of the participants interrupted a session during stimulation. When the device presented malfunctions, it was changed, or the participant was oriented to solve the problem. No cases remained unresolved.
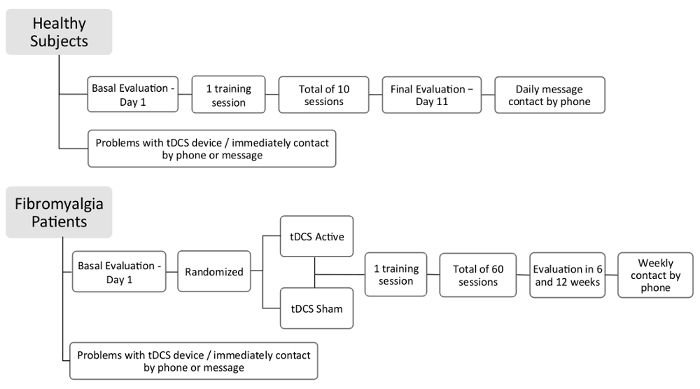
Figure 1: Flow of study
Details of study design – healthy subjects and fibromyalgia patients.
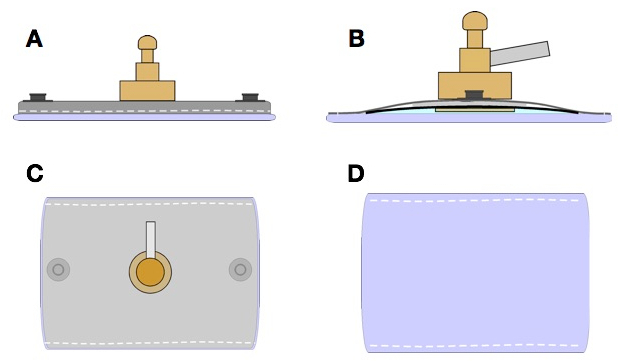
Figure 2: Schematic drawing of the electrode construction
(A) Right side view.(B) Left side view.(C) Upper view.(D) Bottom view.
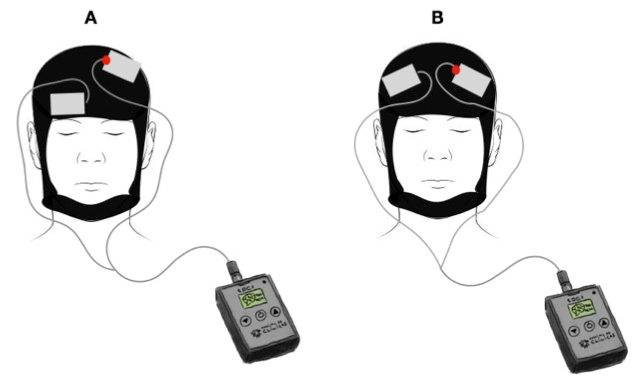
Figure 3: Stimulation area
(A) Area of stimulation healthy subjects: anodal stimulation over left M1 and cathodal stimulation over contra-lateral supra-orbital area. (B) Area of stimulation FM patients: DLPFC area, with anodal stimulation over left side.

Figure 4: Screen sequence
(A) Opening screen. (B) Battery status. (C) Date and time. (D) Command "wear the cap". (E) Command "inject saline". (F) Command to start the session. (G) Beginning of the stimulation.
Figure 5: Saving the session when stimulation ceases
After the end of the stimulation, the tDCS device automatically powers off and stimulation ceases, saving the session.
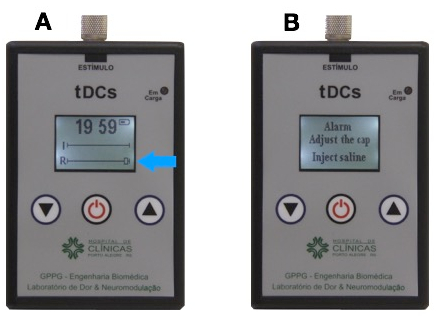
Figure 6: Alarm warning screen
(A) High resistance. (B) Alarm warning: "adjust the cap and inject extra saline".

Figure 7: Shut down screen if the session needs to be stopped during stimulation
If the session needs to be stopped during stimulation, the central button can be pressed for a few seconds, followed by pressing the switch off button and a message "Shut down?" will appear.
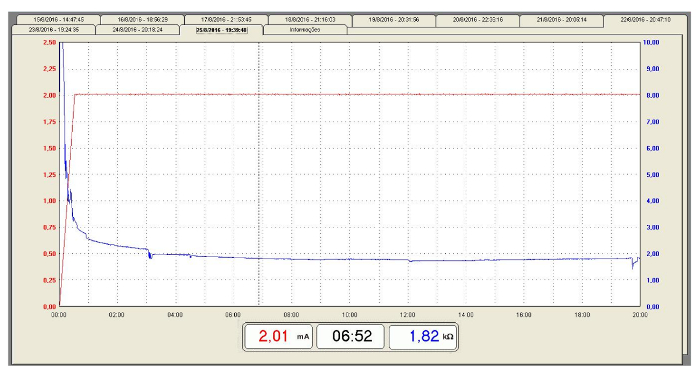
Figure 8: Typical curves of current intensity versus contact impedance during a tDCS session
An example of current intensity and contact impedance curves (healthy subject). Current intensity (mA): Red line. Resistance (kΩ): Blue line. Please click here to view a larger version of this figure.
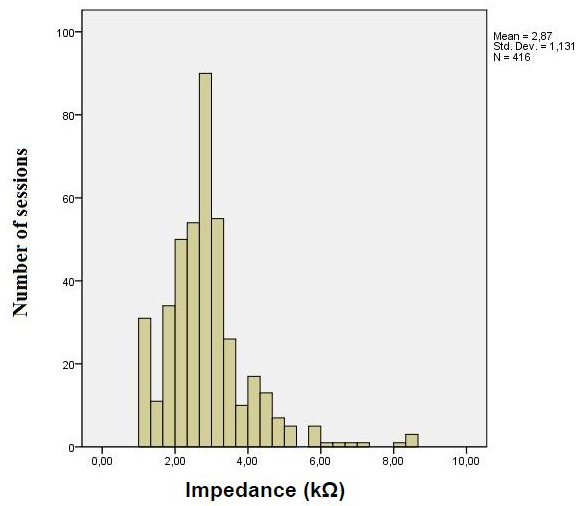
Figure 9: Contact Impedance
Mean of contact impedance – healthy subjects and the fibromyalgia patients active tDCS (n= 416).
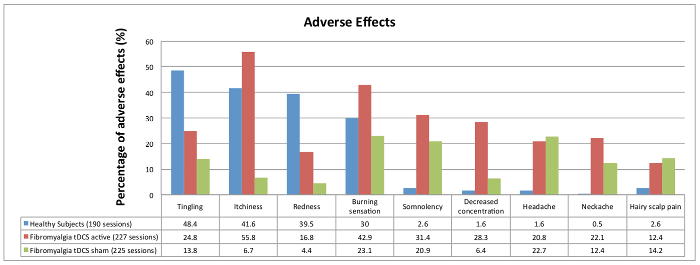
Figure 10: Results of adverse effects
Comparison between groups of participants: healthy subjects (HS), active tDCS fibromyalgia patients (FM active) and sham tDCS fibromyalgia patients (FM sham). Please click here to view a larger version of this figure.
Discussion
The device presented an adequate user interface via the LCD (liquid crystal display) and was user-friendly and easy to use. Our home-based tDCS equipment proved to be suitable for long-term use since adherence was higher than 90% even when participants were instructed to perform 60 home-based sessions. This enables the reproducibility of research conditions13,21 necessary for the idealization and confidence in studies with this device.
It is challenging to maintain adherence when research involves consecutive sessions without intervals on weekends. This fact is one of the hypotheses raised to justify why healthy participants had slightly lower adherence than fibromyalgia patients. Also, a rationale to explain the current results may be that patients with chronic pain have the possibility of pain relief as a motivator, which does not occur in healthy subjects.
The use of home-based tDCS allows participants to choose what time of day to receive the treatment, therefore accommodating patients' schedules. Also, other research protocols comparing different times of tDCS application can be devised using this device, linking to other areas of study, such as chronobiology of pain, for example. Besides, the device proved to be safe for use with no real-time supervision of the sessions due to its security system. The device can be preconfigured to allow for an interval between sessions, duration of stimulation, intensity, maximum resistance, number of courses and active or sham stimulation predefined by a programmer. Therefore, it is possible to program for daily use of 20 min with a minimal interval of 12 hours between sessions (or other configurations). Thus, researchers can monitor adherence using information kept in the device software. Therefore, our results proved that device features ensure reliability and reproducibility of the stimulation, which is a crucial factor in the development of clinical trials.
Electrode-skin contact impedance is an important variable to ensure the safety of tDCS use16,21. High impedance may be related to poor coupling between the electrode and the skin or dryness of the electrode's sponge. These situations may increase current density and lead to skin lesions22. In our study, the use of 6 mL of saline solution was sufficient to guarantee low contact impedance and was adequate and similar in both groups.
The adverse effects produced by our device do not differ from those reported in ambulatory use. Thus, our home-based tDCS resembles traditional equipment, and it is feasible, reliable and safe for use at home in consecutive sessions for long-term therapies, allowing the possibility of running more extensive clinical trials with larger samples.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank the collaborators from the department of Biomedical Engineering at the HCPA for their assistance and support. Acknowledgments to Mário Eugênio Saretta Poglia, Francisco Mauro and Cássia Garzela for the photos and filming and Emillie Pinheiro and Ketlin Bazzo for their participation in the video and photos. We also thank the União das Américas Faculty, which provided the place for video recording. This research was supported by grants and material support from the following Brazilian agencies: Committee for the Development of Higher Education Personnel (CAPES), National Council for Scientific and Technological Development (CNPq), Postgraduate Program in Medical Sciences at the School of Medicine of the Federal University of Rio Grande do Sul, Postgraduate Research Group at the Hospital de Clínicas de Porto Alegre – FIPE HCPA and FINEP (Research in Biomedical Engineering) – 02/2013: 04.13.0465.00.
Materials
| Fisiologic solution 0.9% | Eurofarma | Sterile and pyrogenic solution, trilaminated packaging, 500 mL, transparent, two equal nozzles for the introdution of the equipment and the needle, protective sealing, registration at the ministery of health – 1.0043.1047.004-6 | |
| Vegetable sponge | Scotch-Brite 3M | Vegetable sponge cloth, package with a unit of 18×22 cm. The vegetable cloth is a composite material of regenerated cellulose, cotton, dimethyl benzylammonium chloride and magnesium chloride (fungicidal substance). It has great absorption power of liquids in general. Available in colors: blue, pink and yellow. | |
| Serynge | Advantine | 10 mL Plastic Serynge | |
| Electrodes | Three layers electrode: Flexible Vinyl material, conductive rubber and vegetal sponge | ||
| Cap | Neoprene of 4 mm thickness – Manufactured by Biomedical Engineering Department of Hospital de Clínicas de Porto Alegre | ||
| tDCS device | Manucfated by Biomedical Engineering Department of Hospital de Clínicas de Porto Alegre |
References
- Brunoni, A. R., et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. British Journal of Psychiatry. 10, 1-10 (2016).
- Brunoni, A. R., et al. Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression. New England Journal of Medicine. 376, 2523-2533 (2017).
- Boggio, P. S., Zaghi, S., Lopes, M., Fregni, F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. European Journal of Neurology. 15, 1124-1130 (2008).
- Fenton, B. W., Palmieri, P. A., Boggio, P., Fanning, J., Fregni, F. A preliminary study of transcranial direct current stimulation for the treatment of refractory chronic pelvic pain. Brain Stimulation. 2, 103-107 (2009).
- Fregni, F., et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 122, 197-209 (2006).
- Fregni, F., et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis and Rheumatism. 54, 3988-3998 (2006).
- Valle, A., et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham- controlled longitudinal clinical trial. Journal of pain management. 2, 353-361 (2009).
- Lindenberg, R., et al. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 74, 280-287 (2010).
- Valiengo, L. C. L., et al. Transcranial direct current stimulation for the treatment of post-stroke depression: results from a randomised, sham-controlled, double-blinded trial. Journal of Neurology, Neurosurgery, and Psychiatry. , jnnp-2016-314075 (2016).
- DaSilva, A. F., Volz, M. S., Bikson, M., Fregni, F. Electrode positioning and montage in transcranial direct current stimulation. Journal of Visualized Experiments. , e2744 (2011).
- Kasschau, M., et al. Transcranial Direct Current Stimulation Is Feasible for Remotely Supervised Home Delivery in Multiple Sclerosis. Neuromodulation. 19, 824-831 (2016).
- Charvet, L., et al. Remotely Supervised Transcranial Direct Current Stimulation Increases the Benefit of At-Home Cognitive Training in Multiple Sclerosis. Neuromodulation. , (2017).
- Charvet, L. E., et al. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Frontiers in Systems Neuroscience. Frontiers in Systems Neuroscience. 9, 26 (2015).
- Nitsche, M. A., et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimulation. 1, 206-223 (2008).
- Charvet, L. E., et al. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Frontiers in Systems Neuroscience. 9, (2015).
- Kasschau, M., et al. A Protocol for the Use of Remotely-Supervised Transcranial Direct Current Stimulation (tDCS) in Multiple Sclerosis (MS). Journal of Visualized Experiments. , e53542 (2015).
- Lefaucheur, J. P., et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology. 128, 56-92 (2017).
- O’Neill, F., Sacco, P., Nurmikko, T. Evaluation of a home-based transcranial direct current stimulation (tDCS) treatment device for chronic pain: study protocol for a randomised controlled trial. Trials. 16, 186 (2015).
- Antal, A., Kincses, T. Z., Nitsche, M. A., Paulus, W. Modulation of moving phosphene thresholds by transcranial direct current stimulation of V1 in human. Neuropsychologia. 41, 1802-1807 (2003).
- Halko, M., Eldaief, M., Pascual-Leone, A. Noninvasive brain stimulation in the study of the human visual system. Glaucoma. 22, 39-51 (2013).
- Bikson, M., Datta, A., Elwassif, M. Establishing safety limits for transcranial direct current stimulation. Clinical Neurophysiology. 120, 1033-1034 (2009).
- Minhas, P., et al. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. Journal of Neuroscience Methods. 190, 188-197 (2010).

