Disentangling High Strength Copolymer Aramid Fibers to Enable the Determination of Their Mechanical Properties
Summary
The primary goal of the study is to develop a protocol to prepare consistent specimens for accurate mechanical testing of high strength copolymer aramid fibers, by removing a coating and disentangling the individual fiber strands without introducing significant chemical or physical degradation.
Abstract
Traditionally, soft body armor has been made from poly(p-phenylene terephthalamide) (PPTA) and ultra-high molecular weight polyethylene. However, to diversify the fiber choices in the United States body armor market, copolymer fibers based on the combination of 5-amino-2-(p-aminophenyl) benzimidazole (PBIA) and the more conventional PPTA were introduced. Little is known regarding the long-term stability of these fibers, but as condensation polymers, they are expected to have potential sensitivity to moisture and humidity. Therefore, characterizing the strength of the materials and understanding their vulnerability to environmental conditions is important for evaluating their use lifetime in safety applications. Ballistic resistance and other critical structural properties of these fibers are predicated on their strength. To accurately determine the strength of the individual fibers, it is necessary to disentangle them from the yarn without introducing any damage. Three aramid-based copolymer fibers were selected for the study. The fibers were washed with acetone followed by methanol to remove an organic coating that held the individual fibers in each yarn bundle together. This coating makes it difficult to separate single fibers from the yarn bundle for mechanical testing without damaging the fibers and affecting their strength. After washing, fourier transform infrared (FTIR) spectroscopy was performed on both washed and unwashed samples and the results were compared. This experiment has shown that there are no significant variations in the spectra of poly(p-phenylene-benzimidazole-terephthalamide-co-p-phenylene terephthalamide) (PBIA-co-PPTA1) and PBIA-co-PPTA3 after washing, and only a small variation in intensity for PBIA. This indicates that the acetone and methanol rinses are not adversely affecting the fibers and causing chemical degradation. Additionally, single fiber tensile testing was performed on the washed fibers to characterize their initial tensile strength and strain to failure, and compare those to other reported values. Iterative procedural development was necessary to find a successful method for performing tensile testing on these fibers.
Introduction
Currently, significant focus in the field of personal protection is on reducing the mass of the body armor needed for personal protection for law enforcement and military applications1. Traditional armor designs have relied on materials like poly(p-phenylene terephthalamide) (PPTA), also known as aramid, and polyethylene to provide protection against ballistic threats2. However, there is an interest in exploring different high strength fiber materials for their potential to reduce the weight of armor required to stop a specific ballistic threat. This has led to the exploration of alternative materials such as aramid copolymer fibers. These fibers are made by the reaction of [5-amino-2-(p-aminophenyl)benzimidazole] (amidobenzimidazole, ABI) and p-phenylenediamine (p-PDA) with terephthaloyl chloride to form poly(p-phenylene-benzimidazole-terephthalamide-co-p-phenylene terephthalamide). In this study, we examine three different fibers, all of which are commercially produced materials obtained from an industry contact. One is a homopolymer fiber that is made by reacting ABI with p-phenylenediamine to form poly 5-amino-2-(p-aminophenyl)benzimidazole, or PBIA. The other two copolymer fibers examined in this study are expected to be random copolymers with different ratios of PBIA and PPTA linkages3. The relative ratios of these linkages could not be determined experimentally using solid-state nuclear magnetic resonance. These fibers are designated as PBIA-co-PPTA1, PBIA-co-PPTA3 to extend the designations used in a previous publication4. PBIA-co-PPTA3 was not previously studied, but has a similar structure. These fiber systems have also been the focus of several recently granted patents5,6,7.
Superior ballistic resistance of body armor is predicated on the mechanical properties of the materials that comprise it, such as ultimate tensile strength and strain to failure8,9,10. Significant efforts11,12,13 have been focused on examining the long-term stability of polymeric fibers used in body armor by investigating detrimental changes in these mechanical properties after exposure to environmental conditions. The effect of environmental conditions on aramid copolymer fibers has not been the subject of a lot of research3,4. One challenge to studying these materials is the difficulty in disentangling yarns for testing. Prior work by McDonough4 investigated a technique by which water was used to disentangle yarns prior to performing single fiber tensile testing. However, there was no complete understanding on whether the mechanical strength of the fibers was altered by this water exposure. An alternative to disentangling the fibers is to test the mechanical strength of the yarn bundle, however, this requires a large amount of material, and is considered to average the strength of the fibers in the yarn bundle, providing less specific information. The goal of this project is to examine the effect of elevated humidity and temperature on the mechanical properties of aramid copolymer fibers. Thus, it is essential to find an alternative solvent for coating removal and fiber disentanglement that will enable us to distinguish hydrolysis in the fibers due to the environmental exposure from that induced by sample preparation. The preparation of single fibers for testing is further complicated by their small size. In this work, we investigate several common solvents (water, methanol, and acetone) and select acetone as the best choice for the preparation of single fibers for testing. All fibers were rinsed with methanol before further testing. Fourier transform infrared (FTIR) spectroscopy is performed to determine if the coating dissolution and disentanglement step caused any chemical degradation in the material. The detailed video protocol showing the sample preparation steps of disentanglement, chemical analysis, and mechanical testing of copolymer aramid fibers is intended to assist other researchers in developing methodologies for performing similar studies of single fibers in their laboratories.
Protocol
1. Dissolution of Coating on Copolymer Fibers to Aid in Fiber Separation
- Wearing appropriately selected chemically resistant gloves to prevent contamination of the fiber, cut 160 mm to 170 mm from each yarn bundle extracted using ceramic scissors or a fresh steel razor blade. Reserve the remainder of the yarn if needed for further analysis in a labeled container.
- Knot or clamp the ends of the yarn to keep the yarn from tangling when immersed in the solvent.
NOTE: For this study, solvents of wide ranging polarity (from the polarity series) were initially explored. Based on qualitative results, a more in-depth examination was conducted using acetone, water, and methanol. Finally, acetone was selected as the best solvent for fiber separation based on the ease of detangling and the scanning electron microscopy (SEM) results (described later). - Immerse the fiber in 2 mL to 3 mL of the solvent in a labeled Petri dish and cover with the Petri dish lid.
- Allow the yarns to soak in acetone for 30 min, then discard the solvent.
- Repeat steps 1.3 to 1.4 at least two additional times and then allow the solvent to evaporate.
- To remove any acetone residue and to aid in drying, immerse the sample in 2 mL to 3 mL of methanol.
- Allow the yarns to soak in methanol at least 30 min.
- Remove the yarn from the solvent and allow to dry for at least 24 h.
2. Analysis of Coating Dissolution Step by Scanning Electron Microscopy
- Separate the individual fibers with tweezers, which are previously washed using different solvents from the yarn bundle, for analysis under a stereo microscope if necessary.
- Mount the fibers on a stainless-steel stub (1 cm diameter) by adhering them with tweezers onto double-sided carbon tape.
- Coat the fibers with a conductive material such as Au/Pd to mitigate the surface charging effects under the SEM.
- Load the fiber samples into a scanning electron microscope and image them at 2 kV accelerating voltage and 50 pA – 100 pA electron current. Apply charge neutralization settings to counter charging effects where necessary.
3. Analysis of Coating Dissolution Step by Fourier Transform Infrared Spectroscopy
- Cut approximately 30 mm to 40 mm of the washed yarn bundle.
- Obtain an adhesive IR sample card and remove the protective backing.
- While wearing gloves to protect the sample from contamination, slightly twist the fiber bundle to coalesce the sample for analysis and place the sample over the window in the card.
- Prepare the FTIR for analysis according to the manufacturer's specifications. Turn on the purge gas, fill the detector with liquid nitrogen, and install the ATR accessory using the magnetic alignment plate in the sample compartment.
- Program the parameters for number of scans and instrument resolution in the advanced measurement tab of the instrument software, in this case, 128 scans are averaged at a resolution of 4 cm-1.
- Clean the window of the ATR accessory with a low lint wipe and methanol.
- Collect a background by pressing the collect background button in the basic measurement window of the software with the parameters selected in step 3.5.
- Align the fiber sample over the window in the ATR accessory, using the microscope and video monitor to help position the fiber.
- Collect a sample spectrum by pressing the collect sample button in the basic measurement window of the software using the parameters selected in step 3.5.
- Repeat steps 3.6-3.9, collecting at least 3 spectra per sample until all samples have been analyzed.
4. Analysis of Fibers by Wide Angle X-Ray Scattering
- While wearing nitrile gloves, cut approximately 25 mm of the yarn from the yarn spool using a razor blade.
- Center each bundle of the yarn over the 6.25 mm inner hole of a 25 mm stainless steel washer.
- Tape the yarn bundle to the washer to hold it in place using cellophane tape.
- Repeat steps 4.1 to 4.3 for the other two types of yarn.
- Tape the washers containing the yarn bundles to a stainless-steel sample holder block (which contains metallic rods for positioning) as shown in Figure 1. The fibers should be in the vertical configuration for analysis.
- Mount a silver behenate control sample to the sample holder block in the same position as the washers.
- Open the door to the instrument and mount the sample holder block to the analysis stage using the magnetic alignment system.
- Close the door to the sample holder chamber and activate the vacuum pump to evacuate the sample analysis chamber. Monitor the vacuum gauge mounted next to the instrument until the vacuum reaches approximately 1600 Pa.
- Open the instrument software, activate the beam, and perform a horizontal scan to determine the x-location of each sample on the sample holder.
- After identifying x-location of each sample, perform a vertical scan to optimize the y-location to obtain the maximum signal intensity for each sample.
- Once the x and y-locations are determined, begin the measurement by analyzing the silver behenate control sample to determine the distance between the sample and each detector.
- Analyze the first fiber sample using a 10 min exposure time.
- Repeat step 4.13 two additional times for a total scan time of 30 min.
NOTE: This protocol is used instead of one long 30 min scan case because there are issues with the sample exposure to minimize wasted instrument time. - Average the 3 scans to obtain the final result using the average function in Fit 2D software.
- Repeat steps 4.13-4.15 for each additional sample.
5. Yarn Disentanglement and Preparation for Tensile Testing
- Obtain a 30 cm x 30 cm or larger transparent plastic board (polycarbonate sheets are used in these experiments) that can be placed on a dark background, or a dark plastic board of the same dimensions.
- Cut pieces of low tack masking tape (approximately 10 mm 5 mm) and have them available for the following steps. Perform this step on a glass surface and cut the tape with a razor blade.
- Tape both ends of a 20 mm gauge rectangular paper template to the plastic board so that it lies completely flat.
NOTE: 20 mm is selected as the optimal gauge length for these tests based on previous work and the available jaw separation of the instrument. - Wearing nitrile gloves to prevent contamination, cut approximately 70 mm to 80 mm of rinsed yarn and place it on a glass slide or other clean surface (Figure 2a-b).
- Using a stereo microscope to assist disentanglement, carefully remove a single fiber from the yarn using tweezers. Take care to avoid snagging or damaging the fiber during this process. Discard any fibers that are damaged (Figure 2c).
- Place a single fiber on top of the paper template, making sure that the fiber is aligned with the markers on the template (Figure 2d-f).
- Tape both ends of the fiber to the board. In order to improve the visibility of the fiber, put a dark background underneath the transparent plastic board or use a black plastic board. The fiber should lay straight and slightly taught across the template (Figure 2f).
- Repeat steps 5.3 to 5.7 until approximately 35 to 45 fibers are mounted on separate paper templates for each type of fiber. In this case, there are three types of fibers: PBIA-co-PPTA1, PBIA, and PBIA-co-PPTA3.
- Once all fibers are taped to the plastic board, add one small drop of cyanoacrylate adhesive to each end of the fiber aligned to the paper template. Leave 1 cm free of glue at the ends of the paper templates for gripping during tensile testing.
NOTE: Cyanoacrylate was found to be the best adhesive for this material, an unsuccessful attempt with a 24 h cure epoxy is shown in the Representative Results. - Allow the adhesive to cure for at least 24 h before testing.
6. Single Fiber Tensile Testing
- Determine the gage length and the rate of extension that provides the most consistent results for the specimen of interest. These parameters may be dictated by the amount of available sample and by the limitations of the experimental setup.
- Prepare the instrument for testing by installing tensile grips and calibrating the gap.
- Program the instrument to move the grips to provide a gap of 30 mm, which is the gage length selected based on the size of the paper template and the 10 mm space left at each end for the jaw.
- Loosen the grip faces to create a gap for loading the paper template that contains the single fiber.
- Move one of the samples prepared in step 5 to the instrument. Using gloved hands, a small spatula, and tweezers, feed the template through both grips, using the marks on the template to assist the placement. Make sure that the glue is outside of the grip area.
- Gently align and close the top grip face, while still supporting the fiber so that it does not slide down.
- Tighten the top and bottom screws with a torque wrench until the screws are just tight.
- Repeat step 6.7 for the bottom screws.
- Tighten the screws on the upper and lower grips using a torque wrench. Take care to tighten the screws in a cross pattern to balance the load on the fiber.
NOTE: The appropriate torque to use may vary and must be experimentally determined. 30 cN·m was used in these experiments. - Trim both sides of the paper template with scissors.
- Program the instrument to perform the tensile test at a constant rate of extension of 0.0125 mm/s, monitor the display and stop the test when the fiber has broken.
- At the end of the test, remove the fiber from the grips by loosening the grip faces. Observe the break location and preserve the broken fiber in a labeled container for further analysis.
NOTE: Fibers that break at the grip face are discarded from analysis as "jaw breaks" as described in ASTM D3822. - Return the gap to 30 mm and repeat steps 6.4-6.12 until all samples are tested.
- Save the broken fiber fragments in the template for further microscopic analysis.
Representative Results
The copolymer aramid fibers studied here are difficult to separate from yarn bundles into individual fibers for testing. The fibers are entangled and coated with processing chemicals that make them very difficult to separate without damaging the fibers. Figure 3 shows the structural morphology of fibers within a yarn. Even as part of a larger bundle, the fiber surfaces show extensive roughness and tears that are likely caused by strong adhesion to adjacent fibers. In previous work by McDonough4, et al., water was used to separate the fibers prior to tensile testing, however, the chemical analysis of fibers prepared using this method raised important questions regarding the sample preparation and its effects on mechanical properties. In the first part of this work, the effectiveness of three different solvents (chosen by the elimination from the entire polarity range of solvents), including water, are compared using SEM to examine the effect of different washing protocols on the physical appearance of the separated fibers. The water and acetone immersed fibers were rinsed in methanol after washing to remove any solvent residue and aid in drying the water immersed fibers more quickly. Figure 2 shows an overview of the fiber bundle disentanglement procedure. The washed fibers are also compared against as received fibers that were separated from the yarn bundle without any further sample preparation. Resulting micrographs are presented in Figure 4, Figure 5, Figure 6.
In Figure 4a, note that the physical damage to the PBIA-co-PPTA1 fiber in the form of fibrillation when the "dry" fiber was separated without the use of any solvents. Also note the presence of flaking and longitudinal grooves on the fiber surfaces due to immersion in water (Figure 4b), which could be indicative of degradation mechanisms such as hydrolysis, or caused by incomplete removal of the chemical coating from the fiber. These features are moderately observed in the methanol (Figure 4c)) and acetone (Figure 4d) immersed fibers, but the acetone immersed fiber appears to have the least solvent-induced damage and predominantly exhibits a clean and smooth surface. As the primary goal of the study was to develop a methodology to separate individual fibers for mechanical testing while ensuring minimal fiber damage (physical or chemical) during the separation process, traces of residual chemical coating can be observed in SEM images of the washed fibers (Figure 5a). The goal was not to completely dissolve the coating, just enough to be able to separate the yarns with minimal damage.
In Figure 5a, the physical damage to the PBIA-co-PPTA3 fiber in the form of longitudinal grooves and fibrillation are observed especially at the fiber edges of the "dry" fiber separated without any immersion. The fiber immersed in water (Figure 5b) also shows some damage to the edges where it appears to have been adhered to an adjacent fiber prior to the separation. The methanol (Figure 5c) and acetone (Figure 5d) immersed fibers both show much less fibrillation, but as observed previously, the fibers immersed in acetone qualitatively appear to have lesser surface artifacts than the other fibers.
In Figure 5, the physical damage to the dry PBIA is observed to be less severe than the other two fibers, but there is some evidence of longitudinal grooves along the fiber in the lower part of the image (Figure 6a). The fiber immersed in water (Figure 6b) shows minor damage at the edges caused by strong attachment to an adjacent fiber. The methanol and acetone immersed fibers (Figure 6c-d) show similar physical characteristics as the water immersed fiber.
To further examine the effect of the acetone rinsing on the fibers, FTIR spectroscopy was performed. The result of this analysis is presented in Figure 7. Some intensity changes are observed after washing, but no major changes in spectra indicative of chemical degradation (e.g., changes in the OH/NH region around 3300 cm-1 or the formation of a carbonyl peak around 1700 cm-1) are observed. Therefore, the acetone rinsing procedure was selected as the best fiber preparation method for the rest of the study.
The next step in this study was to determine the best method of tensile testing single fibers with the existing equipment setup. An effort was made to directly test fibers by mounting fibers in the grips and performing the test. As this method requires minimal sample preparation, and the samples exhibited no slippage from the grip, this was regarded as the most rapid way to perform the test. However, most of the fibers tested in this manner broke right at the grip face, a phenomenon known as a "jaw break". As described in ASTM D382214, this result indicates that the test is invalid. Therefore, based on the recommendations of the ASTM D3822 standard, the single fibers were then mounted on a cardstock template before testing.
The fibers were adhered to the cardstock templates using either epoxy or cyanoacrylate and allowed to cure for at least 24 h before testing. Two types of epoxy adhesive were tested, one requiring a 24 h cure and the other requiring a 1 h cure. Nearly all samples adhered to the paper templates with the epoxy adhesive (both the slow and the fast cure) exhibited an uncharacteristic slipping behavior and jagged stress strain curves, as shown by the representative example given in Figure 8a. However, Figure 8b depicts a representative stress-strain curve obtained with the cyanoacrylate adhesive, which is predominantly devoid of sample slippage. A similar behavior was observed in all the fiber systems used in the current study, thereby making cyanoacrylate the most suitable test adhesive to glue the fibers on the templates. Following the success of cyanoacrylate adhesive, all samples were tested according to the recommendations provided in a previous study on single fiber testing of polyethylene14. Overall, the fibers adhered with the cyanoacrylate generally had smooth and continuous stress-strain curves and didn't exhibit significant slippage. While a few fibers failed near the top of the gauge area of the fiber, the use of the template helped us exclude these fibers effectively.
After settling on the cardstock template and cyanoacrylate glue method, the tensile strength and strain to failure of all three fibers could be measured. The results of these tests are presented in Table 1. For each fiber type, 35 samples were tested, and the fourth column of the table reports the successful number of tests in each data set (between 15 and 26 tests). A nominal diameter of 14 µm was used to compute the tensile strength for all fibers, based on previous work and measurements from micrographs of more than 30 fibers. SEM imaging of the failed fibers (Figure 9) indicates that all the fibers undergo brittle fracture resulting in fibrillation. As the fibers used in the current study are mostly non-crystalline (as shown by the wide angle X-ray scattering (WAXS) measurements in Figure 10), minimal plastic deformation is observed in SEM images of these fiber cross-sections, with no evidence of necking.
The mounting configuration for the WAXS analysis is shown in Figure 1, and the results of this analysis are presented in Figure 10. The WAXS analysis indicated that the equatorial diffraction scattering of the PBIA, PBIA-co-PPTA1, and PBIA-co-PPTA3 fibers was very similar, consisting of a broad asymmetrical peak at a 2θ of about 22°. This is indicative of a non-crystalline structure with an absence of orientation in the plane perpendicular to the polymer chain axis. However, the diffraction pattern and the diffractograms of the meridional scattering revealed the presence of two major Bragg peaks at 2θ angles of about 26° and 28° (Figure 10). The strongest peak of the two is at a 2θ of 28° with a d-spacing of about 0.31 nm, and is also present on the meridional diffraction scans of typical PPTA fibers15. The fact that these Bragg peaks on the PBIA, PBIA-co-PPTA1, and PBIA-co-PPTA3 fibers are very weak is indicative of the very low amount of the PPTA linkages in the copolymer structure of these fibers. Additionally, the PBIA-co-PPTA1 and PBIA-co-PPTA3 fibers diffractograms of the meridional scattering revealed the presence of two weak peaks at 2θ angles of about 18° and 21°. Ultimately, these fibers show a very low degree of crystallinity along the chain axis.

Figure 1: An illustrative process to show the methodology for mounting fibers on washers for analysis by WAXS. The silver behenate control sample is not pictured in this photograph. Please click here to view a larger version of this figure.
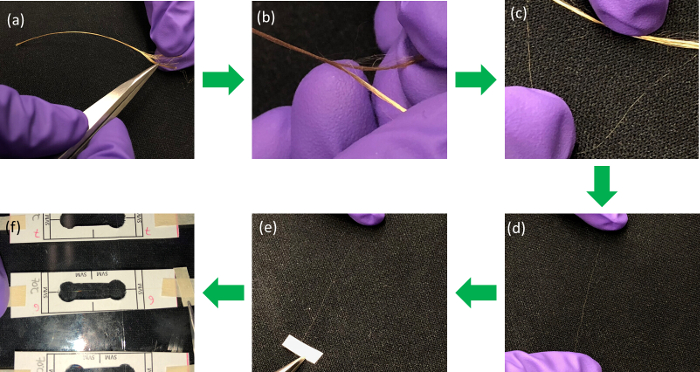
Figure 2: An illustrative process to disentangle a single fiber from the yarn bundle for tensile testing. Please click here to view a larger version of this figure.
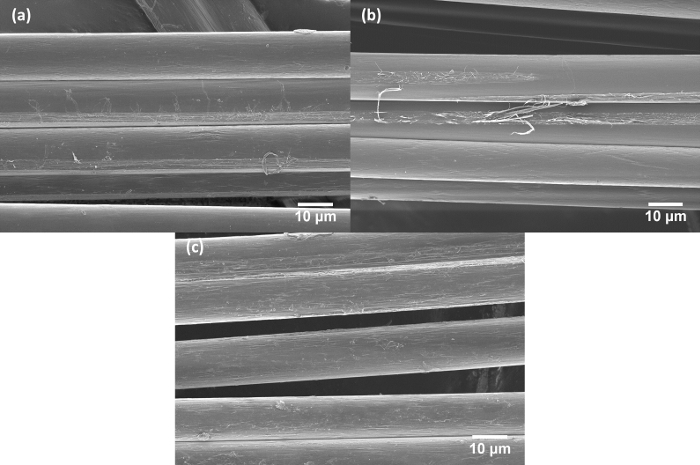
Figure 3: Representative scanning electron micrographs of the fibers within a fiber yarn. (a) PBIA-co-PPTA1, (b) PBIA-co-PPTA3, and (c) PBIA. Please click here to view a larger version of this figure.
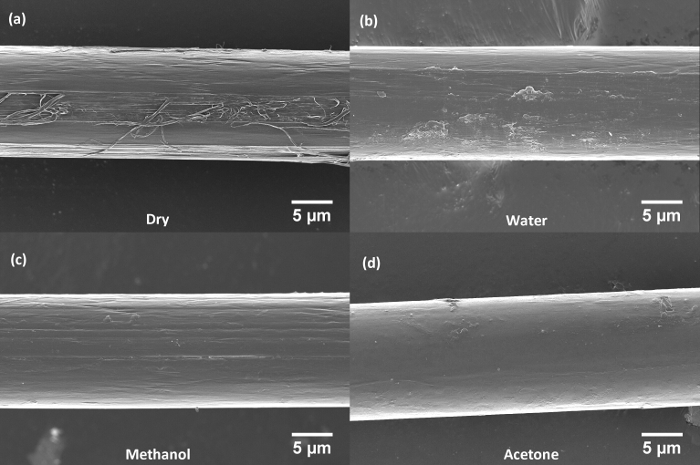
Figure 4: Representative scanning electron micrographs of separated single fibers of PBIA-co-PPTA1 after treatment. (a) Separated dry fiber (no immersion), (b) fiber after immersion in water, (c) fiber after immersion in methanol, and (d) fiber after immersion in acetone. Please click here to view a larger version of this figure.
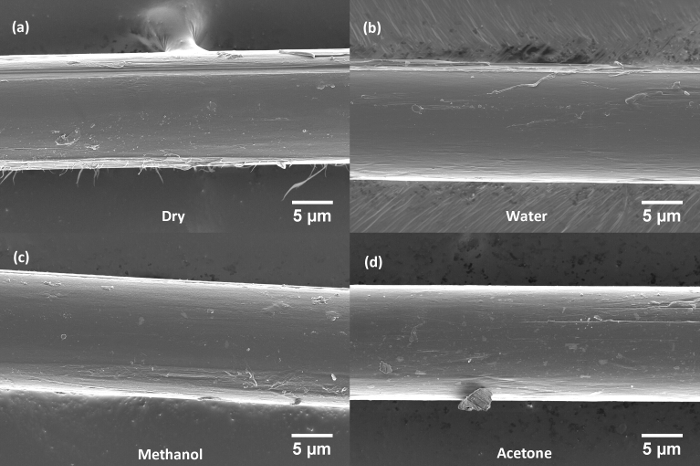
Figure 5: Representative scanning electron micrographs of separated single fibers of PBIA-co-PPTA3 after treatment. (a) Separated dry fiber (no immersion), (b) fiber after immersion in water, (c) fiber after immersion in methanol, and (d) fiber after immersion in acetone. Please click here to view a larger version of this figure.
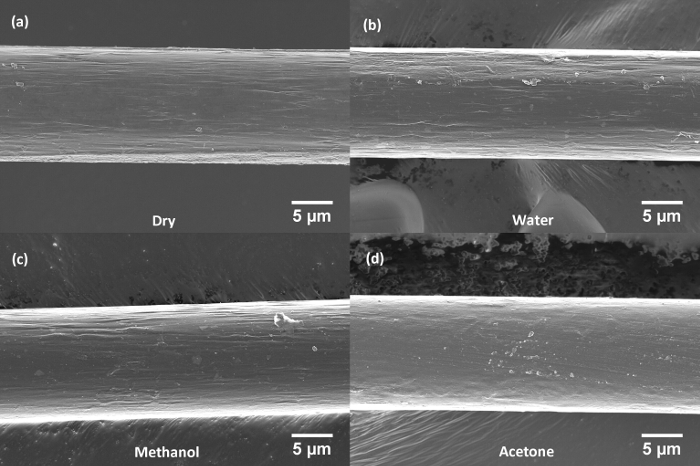
Figure 6: Representative scanning electron micrographs of separated single fibers of PBIA after treatment. (a) Separated dry fiber (no immersion), (b) fiber after immersion in water, (c) fiber after immersion in methanol, and (d) fiber after immersion in acetone. Please click here to view a larger version of this figure.
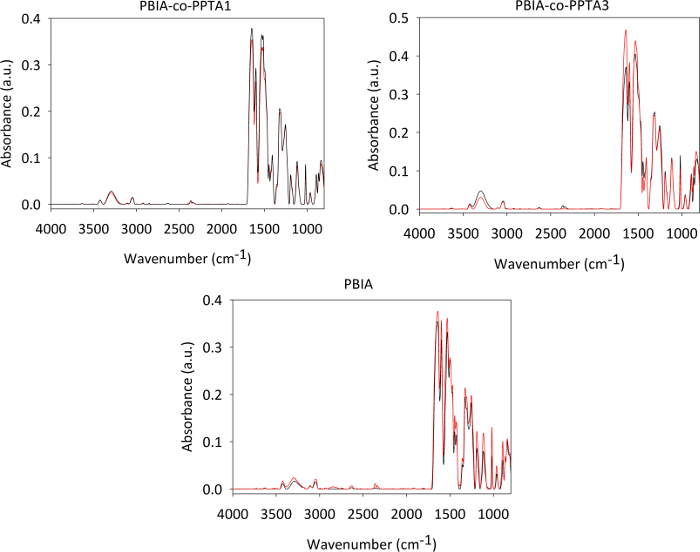
Figure 7: Representative ATR-FTIR spectra of as received dry (black) and acetone washed (red) fibers. Other than slight intensity changes, no major differences indicating chemical changes were observed in the fibers before and after washing. All spectra presented are the average of at least 3 measurements and were collected at a resolution of 4 cm-1. The standard uncertainty in absorbance for this technique is approximately 5%. Please click here to view a larger version of this figure.
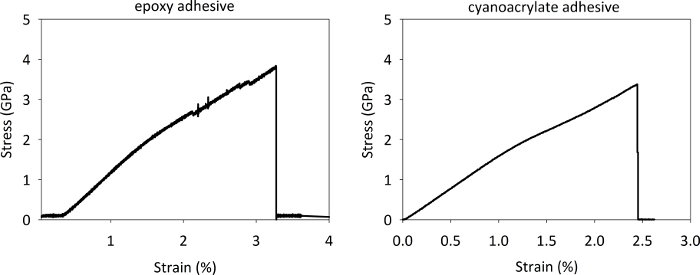
Figure 8: Representative stress-strain curves of PBIA-co-PPTA1 fiber prepared with epoxy adhesive (left) and cyanoacrylate adhesive (right). Note the jagged character of the epoxy curve and the higher strain to failure, which may be representative of slippage in the adhesive. Please click here to view a larger version of this figure.
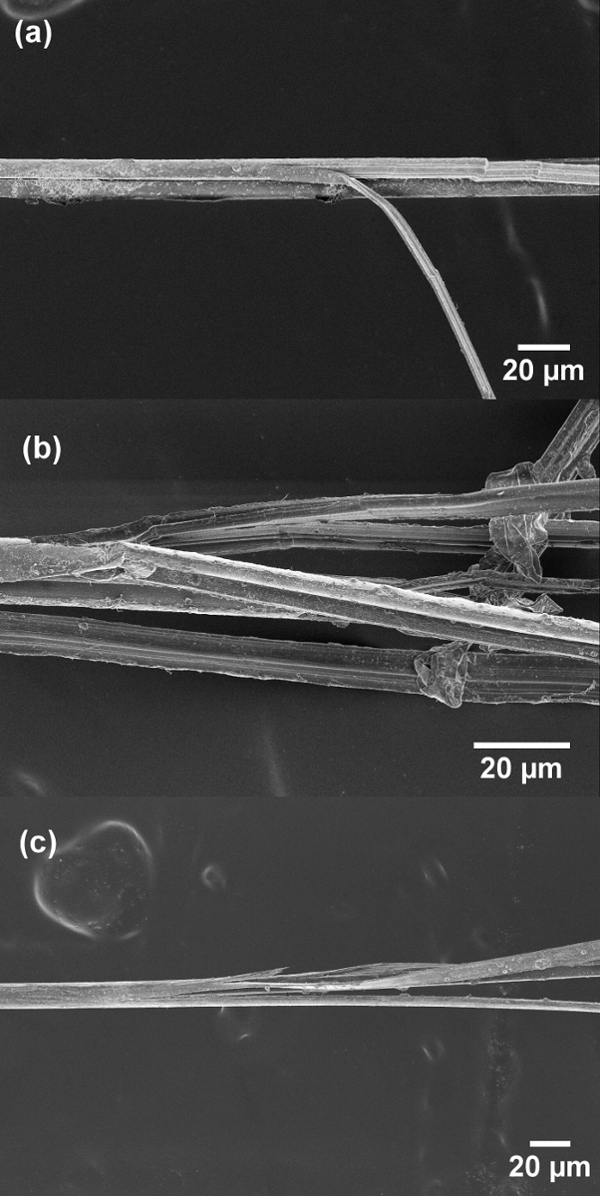
Figure 9: Scanning electron micrographs of failed single fiber cross-sections after acetone treatment: (a) PBIA-co-PPTA1, (b) PBIA-co-PPTA3, and (c) PBIA. All fiber specimens exhibit fibrillation and brittle fracture. Please click here to view a larger version of this figure.
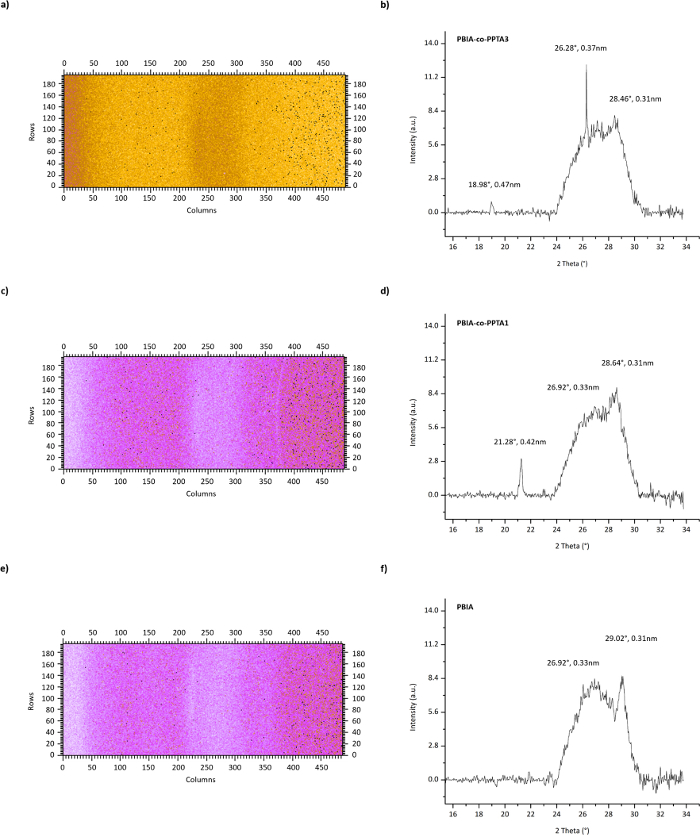
Figure 10: Wide-angle X-ray diffraction patterns of PBIA-co-PPTA3 (a), PBIA-co-PPTA1 (c), and PBIA (e) fibers. Meridional wide-angle X-ray diffractograms of PBIA-co-PPTA3 (b), PBIA-co-PPTA1 (d), and PBIA (f) fibers. Please click here to view a larger version of this figure.
| Fiber type | Tensile Strength (SD) GPa | Strain to failure (SD) % | Modulus (GPa) | Number of samples |
| PBIA-co PPTA1 | 3.26 (0.60) | 2.34 (0.31) | 1.39 (0.11) | 15 |
| PBIA-co-PPTA3 | 3.05 (0.54) | 2.15 (0.30) | 1.38 (0.15) | 26 |
| PBIA | 2.46 (0.45) | 2.46 (0.45) | 1.06 (0.09) | 20 |
Table 1: Mean single fiber tensile properties of acetone washed PBIA-co-PPTA1, PBIA-co-PPTA3, and PBIA. The standard deviation is reported in parentheses next to the value.
Discussion
The method described herein provides an alternate solvent-based protocol for removing coatings from aramid copolymer fibers without using water. Two previous studies3,4 showed the evidence of hydrolysis in the fibers of this chemical composition, with exposure to water vapor or liquid water. Avoiding hydrolysis during the sample preparation is critical for the next phase of experiments where these sets of fibers will be examined for their susceptibility to ageing due to hydrolysis from exposure to warm and humid environments.
Separating and mounting the fibers is the most critical step in this experimental protocol. Extreme care must be taken to isolate only a single fiber (as the fibers can stick together), without damaging them with rough handling during the mounting steps. The selection of the proper adhesive is also critical, as evidenced by the poor results with the epoxy adhesive as compared to the cyanoacrylate. Previous work has also shown that selection of the proper adhesive for a given fiber can be a significant experimental challenge16. This was especially necessary for the PBIA-co-PPTA3 sample, where the protocol used herein resulted in some tests which must be excluded from analysis. However, this result will provide a guideline for future experimentation in the form of preparing additional samples for aging studies.
McDonough and coworkers4 reported both wet and dry tensile strengths and strain to failures for two of the three fibers examined in this study. They used a different experimental apparatus and were able to successfully directly grip the fibers in this apparatus instead of using a template. When the wet testing results from McDonough's work are compared to these results, the PBIA showed a statistically significant difference in the strength properties. The average tensile strength of the PBIA sample was about 0.5 GPa higher than that reported by McDonough4. FTIR results on the wet PBIA samples used in this previous study3 showed evidence of hydrolysis, which can cause a reduction in the strength. Further, the inability to conduct large-scale diameter measurements with high accuracy limits us to using average measurements across a fiber cross section, which may skew our results. While the ultimate goal of our research is to examine the changes in tensile strength relative to the unaged sample due to ageing, our results could be improved by directly measuring the diameter of each fiber instead of relying on a nominal value to obtain a more accurate tensile strength. Improvements in this aspect of our method will be incorporated for future work.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge Dr. Will Osborn for helpful discussions and assistance with preparation of the cardstock template.
Materials
| Stereo microscope | National | DC4-456H | Digital microscope |
| RSA-G2 Solids Analyzer | TA Instruments | Dynamic mechanical thermal analyzer used in transient tensile mode with Film Tension Clamp Accesory | |
| Vertex 80 | Bruker Optics | Fourier Transform Infrared spectrometer used to analyze results of washing protocol, equipped with mercury cadmium telluride (MCT) detector. | |
| Durascope | Smiths Detection | Attenuated total reflectance accessory used to perform FTIR | |
| Torque hex-end wrench | M.H.H. Engineering | Quickset Minor | Torque wrench |
| Methanol | J.T. Baker | 9093-02 | methanol solvent |
| Acetone | Fisher | A185-4 | acetone solvent |
| Cyanoacrylate | Loctite | Super glue | |
| FEI Helios 660 Dual Beam FIB/SEM | FEI Helios | Scanning electron microscope | |
| Denton Desktop sputter coater | sputter coater | ||
| 25 mm O.D. stainless steel washers with a 6.25 mm hole | 25 mm O.D. stainless steel washers with a 6.25 mm hole | ||
| Silver behenate | Wide angle X-ray scattering (WAXS) standard | ||
| Xenocs Xeuss SAXS/WAXS small angle X-ray scattering system | Xenocs Xeuss | SAXS/WAXS small angle X-ray scattering system equipped with an X-ray video-rate imager for SAXS analysis with a minimum Q = 0.0045 Å-1, detector separate X-ray video-rate imager for WAXS analysis (up to about 45° 2θ) sample holder chamber. | |
| Fit 2D software | Software to analyze WAXS data |
References
- Joseph, A., Wiley, A., Orr, R., Schram, B., Dawes, J. J. The impact of load carriage on measures of power and agility in tactical occupations: A critical review. International Journal of Environmental Research and Public Health. 15 (1), (2018).
- . . High-performance fibres. , (2001).
- Messin, G. H. R., Rice, K. D., Riley, M. A., Watson, S. S., Sieber, J. R., Forster, A. L. Effect of moisture on copolymer fibers based on 5-amino-2-(p-aminophenyl)- benzimidazole. Polymer Degradation and Stability. 96 (10), 1847-1857 (2011).
- McDonough, W. G., et al. Testing and analyses of copolymer fibers based on 5-amino-2-(p-aminophenyl)-benzimidazole. Fibers and Polymers. 16 (9), 1836-1852 (2015).
- De Vos, R. E. T. P., Surquin, J. E., Marlieke, E. J. . US patent. , (2013).
- Lee, K. S. . US patent. , (2014).
- Mallon, F. K. . US patent. , (2014).
- Cunniff, P. M. Dimensionless Parameters for Optimization of Textile-Based Armor Systems. 18th Int Symp Ballist. , 1302-1310 (1999).
- Cuniff, P. M., Song, J. W., Ward, J. E. Investigation of High Performance Fibers for Ballistic Impact Resistance Potential. Int SAMPE Tech Conf Ser. 21, 840-851 (1989).
- Cheng, M., Chen, W., Weerasooriya, T. Mechanical Properties of Kevlar® KM2 Single Fiber. Journal of Engineering Materials and Technolog. 127 (2), 197 (2005).
- Forster, A. L., et al. Hydrolytic stability of polybenzobisoxazole and polyterephthalamide body armor. Polymer Degradation and Stability. 96 (2), 247-254 (2011).
- Forster, A. L., et al. Long-term stability of UHMWPE fibers. Polymer Degradation and Stability. , 45-51 (2015).
- Holmes, G. A., Kim, J. -. H., Ho, D. L., McDonough, W. G. The Role of Folding in the Degradation of Ballistic Fibers. Polymer Composites. 31, 879-886 (2010).
- ASTM International. . ASTM D3822/D3822M-14 Standard Test Method for Tensile Properties of Single Textile Fibers. , 1-10 (2015).
- Levchenko, A. A., Antipov, E. M., Plate, N. A., Stamm, M. Comparative analysis of structure and temperature behaviour of two copolyamides – Regular KEVLAR and statistical ARMOS. Macromolecular Symposia. 146, 145-151 (1999).
- Jenket, D. . Failure Mechanisms Of Ultra High Molar Mass Polyethylene Single Fibers At Extreme Temperatures And Strain-Rates. , (2017).

