Summary
Stimulus-evoked [Ca2+]i signals of individual human sperm are assessed. Motile cells are loaded with Ca2+-sensitive fluorescent dye (AM-ester method) and immobilised in a perfusable chamber. Cells are imaged by time-lapse fluorescence microscopy and stimulated via the perfusing medium. Responses of single cells (or regions) are analysed offline using Excel.
Abstract
Protocol
Sperm from healthy fertile males, with a normal semen analysis, are normally prepared for imaging as follows.
- Semen samples are stored at 37°C for no more than 30 min. Cells are isolated from the seminal plasma by swim up into supplemented Earle’s balanced salt solution (sEBSS; mM: 1.8 CaCl2.2H2O, 5.37 KCl, 0.81 MgSO4.7H2O, 26.2 NaHCO3, 1.0 NaH2PO4.2H2O, 116.4 NaCl, 55.6 D-glucose, 2.73 Na pyruvate, 41.8 Na lactate) supplemented with 0.3% charcoal de-lipidated/fatty acid free Fraction V BSA (quality of the BSA is crucial for successful capacitation of sperm). 1 ml of sEBBS is pipetted into each of a series of 5 ml tubes and gently underlayered with 0.3 ml of semen. After incubation for 1 hour (37°C; 6% CO2) the top 0.7 ml is gently removed from each tube and pooled. 10 μl of the sperm suspension is diluted with 90 μl of 1% (v/v) formalin to immobilize the cells, then sperm are counted in a Neubauer chamber. Cell density in the suspension is then adjusted (with sEBSS) to 6 million cells/ml.
- The sample is then divided into aliquots of 200 μl in loosely-capped tubes and incubated (37°C; 6% CO2) in for 5-6 h to allow capacitation.
- Coverslips (22×50 mm) have previously been treated with poly-D-lysine. 10 μl of poly-D-lysine solution (10% w/v) is applied as a number of small drops to the centre of the coverslip. The poly-D-lysine is then allowed to air dry. This can be on a heated stage and should be to complete dryness. A coverslip is attached with vacuum grease to an enclosed, purpose-built, perfusable, polycarbonate imaging chamber (dimensions 35 mm x 20 mm x 5 mm; capacity ≈ 180 μl) similar to the Warner RC20 chamber .The poly-D-lysine-coated coverslip forms the base of the chamber when the cells are viewed on an inverted microscope and a 12 mm diameter circular coverslip forms the upper surface of the chamber, allowing transmission of light.
- Oregon Green BAPTA1-AM (OGB) or Calcium Green 1-AM are used for labeling cells. OGB is prepared by dissolving in DMSO. Pluronic acid F127 (a detergent) is included in the DMSO to prevent ‘clumping’ of the dye. This can be prepared in the laboratory (100 mg Pluronic in 0.5 ml DMSO), immediately before use, or can be purchased pre-prepared. 20 μl is added to a 50 μg aliquot of OGB (2.5 mg/ml). The vial can then be stored frozen and thawed for later use.
- After capacitation of the sperm (5-6 h in sEBBS, 37°C; 6% CO2) a tube is selected for imaging and 1.2 μl of OGB solution is added, giving a final concentration of 10 μM. The tube is then incubated for a further 40 min, after which the cell suspension is gently injected into the inflow port of the imaging chamber with a p1000 (blue) pipette tip. The chamber is placed in the incubator, poly-D-lysine-coated coverslip side down, for 20 min. Cells tend to swim along the surfaces of the chamber and will adhere to the poly-D-lysine-coated area.
- The chamber is now mounted on the microscope, connected to the perfusion apparatus and perfused at approx 0.5 ml/min. A roller pump feeds saline into the chamber and overflow from the exit port is removed by a suction pump with trap. The preparation is left in the dark for at least 10 min whilst loose cells and extracellular dye are removed by perfusion of the chamber. Temperature stability is crucially important because small fluctuations may not only affect cell Ca2+ homeostasis, but may also affect Kd of the dye. Experiments are normally performed at 25°C, achieved by maintaining the temperature of the dark room at this level. We have found that maintaining room temperature at the required level provides greater temperature and focus stability than using a temperature controlled stage or a thermostatically controlled heater on the saline inflow.
- Cells are viewed under phase contrast (40x or 60x oil-immersion) objective and an area for imaging is selected. The portion of the poly-D-lysine coated area that is near the inlet port is preferred, where saline flow is laminar and consistent. It is also important to use an area where cell density is appropriate (excessive density affects the quality and integrity of data through pickup of signal from adjacent cells) and where cells are adequately attached but flagellar activity is discernible. A phase contrast image is saved. The microscope is then switched to fluorescence mode (excitation 480 nm; emission 540 nm) and exposure time is reduced to the minimum required to obtain a clear fluorescence image (usually 50-200 ms).
- The experiment is started by activating the time-series image acquisition software (IQ). Typically images are acquired at 0.1 Hz and illumination is restricted to the periods of image acquisition only using a software controlled shutter. Much greater image acquisition rates can be used but allowance must be made for problems of bleaching and generation of artifacts due to photo-damage to the cells (see discussion). IQ (and most other acquisition software platforms) will allow real-time graphing of fluorescence during the experiment.
- After a control period of 3-5 minutes duration manipulation of saline constituents (such as [Ca2+]o) and application of drugs is achieved by replacement of saline in the perfusion header. Time of addition of each stimulus is noted so that time of arrival in the imaging chamber can be calculated, providing sufficient accuracy for assessment of the slow responses described here (sampled at 0.1 Hz). For more rapid responses greater accuracy can be achieved by adding stimuli directly to the saline flow through a second inflow port as provided in the Warner RC20 chamber.
- Images are analyzed offline using IQ. Regions of interest (ROIs) are drawn round each cell (or part of cell) and also a cell free area is selected (for automatic background subtraction by the software. The image series is then checked to remove cells which died or underwent acrosome reaction (which appears as a large and rapid increase in fluorescence followed by loss of cytoplasmic dye) during the experiment and those that showed sufficient movement to cause artifacts when analyzed as a time series.
- A time-fluorescence intensity series is then obtained for each ROI and these data are analyzed offline in Excel. Initially fluorescence is normalized to the mean value obtained during the control period, using the equation R = [ (F Frest) / Frest] x 100%,where R is % change in fluorescence compared to the control period,, F is Fluorescence intensity at a time t and Frest is the mean of at least 30 determinations of F obtained during the control period.
Representative Results
Figure 1 shows a series of images from an experiment in which the cells were stimulated with 3 μM progesterone. The upper row and movie 1 show the fluorescence images in grey-scale and the same mages are shown below (and in movie 2) in pseudocolor (lookup table ’16_colors’ in Image J), where ‘cool’ colors indicate low fluorescent intensity and ‘warm’ colors indicate high fluorescence intensity. Cells can be viewed in pseudocolor during the experiment, using lookup tables in IQ, but using grey scale is generally more informative for assessing .whether the cells are well-labeled. The first two images were collected at 0 s and 100 s after commencing recording. Fluorescence should be stable and tends to be strongest in the post acrosomal area and sperm neck. Progesterone entered the imaging chamber at approximately 175 s and the 3rd and 4th images (180 s and 220 s) shows a rapid increase in [Ca2+]i (fluorescence) originating in posterior head and neck region of the cell. Subsequent images are at 290, 360, 740, 990 and 1440 s, showing the decay of the initial [Ca2+]i transient and development of a secondary sustained elevation. Figure 2 shows a spreadsheet analysis, converting raw fluorescence values for each ROI to normalized fluorescence (% change in fluorescence compared to the control period) Figure 3 shows time-normalized fluorescence plots for 3 cells showing ‘typical’ responses to 3 μM progesterone.
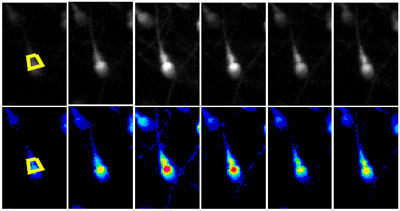
Figure 1. Example data from a cell stimulated with progesterone. A series of fluorescence images in grey scale (upper row) and pseudocolor (lower row) are shown. Time points are 0, 180, 220, 290, 360 and 990s. 3 μM progesterone entered the imaging chamber at 175 s. ROIs are shown in the first panel.
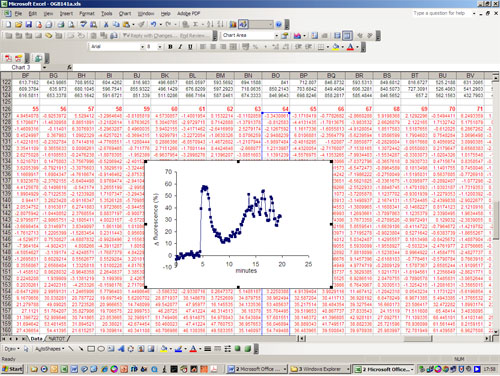
Figure 2. Analysis of single cell fluorescence data in Excel. Figures in black are time series of fluorescence data , arranged vertically. Figures in red (below) are normalized data obtained using the equation given in the text. Graph shows normalized fluorescence-time plot for cell 64 in this experiment which, upon stimulation, shows a transient rise in fluorescence followed by a slow rise and series of small oscillations.
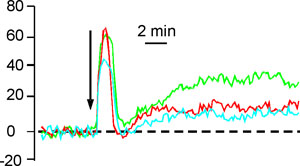
Figure 3. Fluorescence-time traces for 3 individual cells, expressed as % change with respect to control value.. 3 μM progesterone was added at the time marked by the arrow. Dashed line shows mean control fluorescence.
Discussion
When carried out successfully, this technique allows recording of the distribution and kinetics of spontaneous and induced Ca2+ signals in human sperm. Responses can be obtained from a large number of cells (up to 200) which is important since human sperm can show a lot of variation in their spontaneous and stimulated Ca2+ signals. Successful labeling and survival of the cells during recording are highly dependent on sample quality. Poor samples give poor labeling , poor responses and cells may die during recording.
Human sperm are very sensitive to photo-damage and we therefore use the minimal necessary illumination (both intensity and exposure time) in order to maximize cell survival and minimize artifacts due to cell death. A high-sensitivity camera (permitting use of low-intensity illumination) is a great benefit since the camera will pick up weak fluorescence. Back-illuminated, EM-CCD cameras are particularly well-suited, though very expensive. LED illumination (instead of using a xenon or mercury lamp with excitation filter also improves cell survival (Nishigaki et al, 2006).
We do not use Fura-2, despite the obvious advantages of ratio-metric imaging, because Fura requires excitation with UV light and because it is necessary to take two images for each ratio. For data obtained with single wavelength, visible light dyes, normalization of the fluorescence intensity largely compensates for difference in dye loading between cells, but not for dye distribution within an individual cell, and this must be borne in mind. Though single wavelength dyes can, in principle, be calibrated, the accuracy of the technique is poor and we do not attempt to do this.
Whereas the ratio data obtained with Fura-2 (or the emission ratio dye indo), even without calibration, give an acceptably faithful representation of relative changes in [Ca2+]i, the fluorescence intensity of the single wavelength dyes is far from linearly related to [Ca2+]i. Over the most useful part of the saturation curve the relationship for single wavelength dyes usually approximates to logarithmic. We currently use Oregon Green BAPTA-1 (Figure 4) because it is sensitive to small changes in [Ca2+]i close to resting level s (50-100 nM). When [Ca2+]i rises above 1 μM responses will be under-represented in terms of fluorescence change and the dye may saturate. An option for improvement of the technique without resorting to UV excitation is to double load cells with a dye such as Fluo-3 and Fura red. Both can be excited at 488 nM but simultaneous their responses are very different. Recording of emission at 540 and 650 nM provides a ratio which can provide a better method for accurate monitoring of [Ca2+]i (Haughland, 2002) and may be applicable to sperm (Nisigaki et al, 2006).
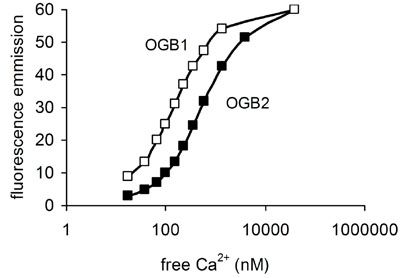
Figure 4. Relationship between free [Ca2+] (nM) and fluorescence emission at peak wavelength (approx 525 nm) for Oregon Green BAPTA-1 and Oregon Green BAPTA-2. OGB1 gives a higher fluorescence at resting [Ca2+] but saturates at lower levels and thus has a smaller useable range. Data for these plots were obtained from the Molecular Probes handbook (Haughland, 2002).
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is funded by the Wellcome Trust, The Medical Research Council, The Royal Society, Birmingham Science City and The Infertility Research Trust. We would like to acknowledge the expert assistance of Cairn Research in constructing and integrating the imaging rigs.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Low light level Camera | QImaging | Rolera XR | ||
| Oregon Green BAPTA-1 | Invitrogen | 06807 | ||
| Imaging Software | Andor | IQ | ||
| Imaging Software | National Institues of Health | Image J | Public domain software | |
| LED illumination system | Cairn Research | Opto LED | ||
| Pluronic F-127 (20% solution in DMSO) | Invitrogen | P3000MP |
References
- Haughland, R. P. . Handbook of Fluorescent Probes and Research Products. , (2002).
- Nishigaki, T., Wood, C. D., Shiba, K., Baba, S. A., Darszon, A. Stroboscopic illumination using light-emitting diodes reduces phototoxicity in fluorescence cell imaging. Biotechniques. 41, 191-197 (2006).

