Summary
Operant drug self-administration and conditioned place preference (CPP) procedures are expansively used in research to model various components of drug reinforcement, consumption, and addiction in humans. In this report, we combined traditional CPP and self-administration methods as a novel approach to studying drug reinforcement and addiction in rats.
Abstract
Animal models of reinforcement have proven to be useful for understanding the neurobiological mechanisms underlying drug addiction. Operant drug self-administration and conditioned place preference (CPP) procedures are expansively used in animal research to model various components of drug reinforcement, consumption, and addiction in humans. For this study, we used a novel approach to studying drug reinforcement in rats by combining traditional CPP and self-administration methodologies. We assembled an apparatus using two Med Associate operant chambers, sensory stimuli, and a Plexiglas-constructed neutral zone. These modifications allowed our experiments to encompass motivational aspects of drug intake through self-administration and drug-free assessment of drug/cue conditioning strength with the CPP test. In our experiments, rats self-administered cocaine (0.75 mg/kg/inj, i.v.) during either four (e.g., the “short-term”) or eight (e.g., the “long-term”) alternating-day sessions in an operant environment containing distinctive sensory cues (e.g., olfactory and visual). On the alternate days, in the other (differently-cued) operant environment, saline was available for self-infusion (0.1 ml, i.v.). Twenty-four hours after the last self-administration/cue-pairing session, a CPP test was conducted. Consistent with typical CPP findings, there was a significant preference for the chamber associated with cocaine self-administration. In addition, in animals undergoing the long-term experiment, a significant positive correlation between CPP magnitude and the number of cocaine-reinforced lever responses. In conclusion, this apparatus and approach is time and cost effective, can be used to examine a wide array of topics pertaining to drug abuse, and provides more flexibility in experimental design than CPP or self-administration methods alone.
Protocol
*=most important steps
- Well-handled male Sprague-Dawley rats are trained to lever press with food reward (45 mg sucrose pellets; Bio-Serv, Frenchtown, NJ).
- *Intravenous catheters are constructed out of stainless steel cannula (Plastics One, VA) and Silastic tubing. Rats undergo a jugular catheterization surgical procedure to allow drug self-administration. Anesthesia consisted of a mixture of oxygen (0.8 l/min; Airgas Southwest, Corpus Christi, TX) and isoflurane (2.5-4%; AErrane, Baxter Healthcare, Deerfield, IL) delivered via a gas delivery system (VetEquip, Inc, Pleasanton, CA). After surgery, catheter patency is maintained by daily flushing with 0.1 ml of a 0.9% saline, heparin and Timentin (antibiotic) solution. Gentamicin, a topical antibiotic, is applied to the skull cap and chest incision to prevent infection. All surgical procedures followed the guidelines for sterile animal surgeries. Surgery supplies were autoclaved and instruments were sterilized in a chemical solution and hot bead sterilizer. Implanted catheters were flushed with methanol, water and air during preparation and then bathed in 70% ethanol solution on the day of surgery. Rats were prepared for surgery by shaving the head and chest area followed application of a betadine solution. After surgery, rats were monitored for several hours before beginning returned to the animal colony.
- Cocaine (NIDA Drug Inventory and Supply and Control Program; RTI International, Research Triangle Park, NC) used in this experiment was dissolved in isotonic saline solution (0.9%) in the appropriate dose concentrations according to animal weights. Cocaine solutions were then filtered through 0.2 um sterile syringe filters prior to daily use.
- *The apparatus is constructed out of two single-lever operant chambers (28 x 22 x 21 cm) equipped with house and stimulus lights and 3 sets of photobeams (Med Associates, St. Albans, VT). Chambers are joined together by a constructed Plexiglas alley (21 x 25 x 25 cm). The alley consists of two black walls with white stripes and two metal walls, a clear Plexiglas top, and a white Plexiglas floor. The two operant chambers have steel grid rod (4.8 mm) floors installed over removable metal cage bottoms. Sensory cues are placed within the operant chambers to create unique, distinguishable environments. The visual cues consist of either white or black felt material attached to the front and back walls and over the cage top. The olfactory cues are oil-based scents (rose or cinnamon) saturating a cotton ball located under the rod floor of the chamber.
- The experimental procedure occurs over multiple days, starting with baseline preference tests (2 days), followed by drug self-administration sessions (8 or 16 days), and ending with a conditioned place preference (CPP) test (1 day).
- *During baseline preference and CPP test procedures, animals have access to both operant chambers. A video camera mounted above the apparatus records the rats’ entrances and exits for each chamber. Photobeams within the chambers detect beam breakages that are used as an index of locomotor activity. During drug self-administration sessions, animals are restricted to a single operant chamber while lever response and locomotor activity (e.g., photobeam breakages) data are recorded with a Med Pentium 100 MHZ computer equipped with Med-PC software (Med Associates, Inc., St. Albans, Vermont).
- Baseline preference measurements are collected on two consecutive days. To begin, the rat is placed in the center alley with access to both operant chambers for a 20-min period. Immediately after placement in the chamber, the video camera, Med-PC software program and timer are simultaneously activated. The baseline preference data are assessed during viewings of the video recordings.
- *Drug self-administration sessions commence the day after the last baseline test. Sensory cues (e.g., white or black wall coverings and rose or cinnamon scents) and a panel to block off access to the Plexiglas center alley are placed into the chamber before the animal. To start the session, the animal is placed within the chamber while tubing from the mounted drug syringe is attached to the animal’s catheter inlet. After closing the operant chamber door, the Med-PC program and timer is started and cocaine access is available for 1 hour. Each lever response results in the infusion of 0.1 ml of cocaine (0.75 mg/kg/inj) or saline solution. At the end of each self-administration session the rat is disconnected from the drug tubing, removed from the chamber and placed in the home cage.
- The day after the last self-administration session, animals undergo a conditioned place preference (CPP) test. The procedure is the same as described for baseline measurements (see 7).
- *Data Assessment: An experimenter blind to group assignments determines the amount of time the animals spend in each compartment by viewing the videotape to assess entrances and exits between the compartments for a 15-min period (beginning 5 min after placement within the apparatus). The same procedure is used to determine baseline preference and CPP test scores.
- *Data Analyses: Differences between baseline measurements and post-conditioning CPP scores are used to determine drug-conditioning effects. Difference scores are calculated by subtracting the amount of time spent in the saline-paired compartment from the amount of time spent in the drug-paired compartment during the CPP test (Number of seconds in Drug-paired compartment) – (Number of seconds in Saline-paired compartment). Statistical analyses on difference score changes from Baseline to Test sessions can be performed to determine effects of drug conditioning.
CPP scores can also be expressed as CPP Difference Scores Percentage (%). In the present study, CPP Difference Score % was calculated first by determining the percent of time spent in each operant chamber using the formula: (Time in Drug (OR Saline) Chamber/Total Time in Each Chamber) * 100 = % Conditioned Place Preference (CPP). The difference score percentage was determined by the formula: (Drug-paired %) – (Saline-paired %) = CPP Difference Score %.
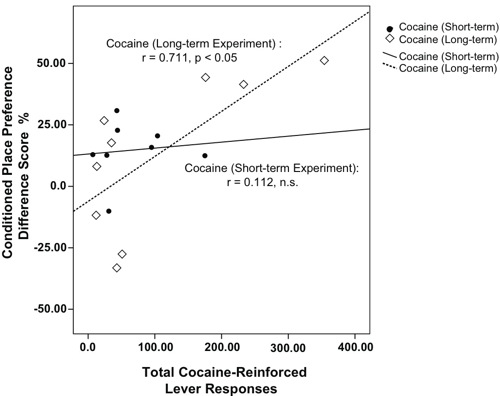
Figure 1. Relationship between Cocaine Intake and Conditioned Place Preference. Pearson’s Correlation analyses determined a significant positive correlation between Total Cocaine-Reinforced Lever Responses and CPP Difference Score % for animals that had self-administered cocaine for eight sessions (e.g., the Long-term group; p < 0.05), but not for animals in the Short-term group (e.g., 4 cocaine sessions). Open diamonds represent individual data points for the Cocaine Long-term (n=9) and filled circles represent the Cocaine Short-term (n= 8) groups. Best-fit lines are dashed for Long-term, and solid for Short-term data points.
Discussion
Operant intravenous drug self-administration and place conditioning procedures are reliable and valid models for studying the neurobiological bases for drug dependence and addiction 1 2 3. Both methods are widely used in pre-clinical drug abuse research and are able to measure the reinforcing properties of abused drugs 4. However, both methods have shortcomings that the new apparatus and method presented here improves upon 5.
One large drawback of traditional CPP procedures is the non-contingent mode of drug administration. This mode of drug intake has different behavioral and neurochemical outcomes than self-administration 6 7 8 9 10 11 , and is not consistent with human drug-taking experiences. Additionally, unlike drug self-administration procedures, CPP paradigms are unable to measure progressive changes in drug motivation as reflected by increased drug intake; a putative turning point in the switch from recreational drug use to uncontrollable drug addiction. However, drug self-administration behavior has interpretive limitations as well. For instance, response rates are often used to infer reward value, but can be directly influenced by motoric effects of the self-administered drug, independent of the drug’s motivational effects. In addition, the number of drug-reinforced responses can also be influenced by presence or absence of drug infusion-associated stimuli 12.
Cocaine is known to be readily self-administered by rats and produce robust conditioned place preferences at a variety of doses and routes of administration 2 13 14. The results from the present study support previous reports of drug reinforcement models. In addition, a significant positive relationship between cocaine-reinforced lever responses and CPP scores was determined in the Long-, but not the Short-term Cocaine group. These results suggest that place conditioning and self-administration are not necessarily isomorphic measures of reward. For instance, it is conceivable that CPP after short-term cocaine exposure reflects acute reinforcing properties typically seen with initial recreational drug use. On the other hand, in rats with more cocaine experience, cocaine intake escalation in correspondence with increasing levels of conditioned reinforcement may indicate progressive changes in drug sensitivity or enhanced rewarding drug effects in certain populations.
This featured method has utility beyond assessing the positive reinforcing effects of drugs. For instance, aversive effects of drugs not present during initial drug use can emerge with extended drug exposure (e.g., conditioned place aversions, or CPA) and would be detectable using this technique. Additional uses include the potential to screen for subpopulations most sensitive to cocaine-cue associative learning, to assess experience-mediated changes in cocaine-motivated behaviors, and to detect enduring drug-conditioned effects during drug abstinence and/or cued reinstatement. In conclusion, this apparatus and approach is time and cost effective, can be used to examine a wide array of topics pertaining to drug abuse, and provides more flexibility in experimental design than CPP or self-administration methods alone.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Leah McAleer for her support in conducting this experiment and her help in the poster presentation at the 2009 Society for Neuroscience Meeting and this video production, along with Mohamed Abdalla and Allison Ahrens. We also appreciate Rosie Maddox, Rachel Chavana, and Linda Ju for their assistance in data collection and analyses for this experiment. This project was supported by NIH/NIDA Grant 3R01DA014640-05S1 (C.L.D), The Waggoner Center for Alcohol and Addiction Research Jones Fellowship and NIH/NIAA Training Grant AA07471 (A.A.F). Cocaine HCl was generously supplied by the NIDA Drug Inventory and Supply and Control Program.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Operant Conditioning Behavior (Drug Self-administration) Test Package for Rat | Med Associates, Inc | MED-008-CT-B1 | ||
| Infrared Source and Detector (Photobeams) | Med Associates, Inc | ENV-253SD ENV-253 |
||
| Med PC Software | Med Associates, Inc | SOF-735 | ||
| Single speed syringe pump | Razel Scientific Instruments | Model R-E | ||
| 45 mg sucrose pellets | Bio-Serv | F0042 | ||
| Catheter cannula | Plastics One | C313G-5UP | ||
| Cocaine | RTI International |
References
- Sanchis-Segura, C., Spanagel, R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 11 (1), 2-2 (2006).
- Tzschentke, T. M. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 12 (3-4), 227-227 (2007).
- Koob, G. F., Bloom, K. D. . Psychopharmacology: the fourth generation of progress. , 759-759 (1995).
- Bardo, M. T., Bevins, R. A. Conditioned place preference: what does it add to our preclinical understanding of drug reward. Psychopharmacology (Berl). 153 (1), 31-31 (2000).
- Panlilio, L. V., Goldberg, S. R. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 102 (12), 1863-1863 (2007).
- Stefanski, R. Active versus passive cocaine administration: differences in the neuroadaptive changes in the brain dopaminergic system. Brain Res. 1157, 1-1 (2007).
- Miguens, M. Differential cocaine-induced modulation of glutamate and dopamine transporters after contingent and non-contingent administration. Neuropharmacology. 55 (5), 771-771 (2008).
- Palamarchouk, V., Smagin, G., Goeders, N. E. Self-administered and passive cocaine infusions produce different effects on corticosterone concentrations in the medial prefrontal cortex (MPC) of rats. Pharmacol Biochem Behav. 94 (1), 163-163 (2009).
- Ciano, P. D. i., Blaha, C. D., Phillips, A. G. Conditioned changes in dopamine oxidation currents in the nucleus accumbens of rats by stimuli paired with self-administration or yoked-administration of d-amphetamine. Eur J Neurosci. 10 (3), 1121-1121 (1998).
- Twining, R. C., Bolan, M., Grigson, P. S. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behav Neurosci. 123 (4), 913-913 (2009).
- Moolten, M., Kornetsky, C. Oral self-administration of ethanol and not experimenter-administered ethanol facilitates rewarding electrical brain stimulation. Alcohol. 7 (3), 221-221 (1990).
- Schindler, C. W., Panlilio, L. V., Goldberg, S. R. Second-order schedules of drug self-administration in animals. Psychopharmacology (Berl). 163 (3-4), 327-327 (2002).
- O’Dell, L. E., Khroyan, T. V., Neisewander, J. L. Dose-dependent characterization of the rewarding and stimulant properties of cocaine following intraperitoneal and intravenous administration in rats. Psychopharmacology (Berl). 123 (2), 144-144 (1996).
- Liu, Y., Roberts, D. C., Morgan, D. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 22 (1), 195-195 (2005).

