Preparation of Hard Palm Seeds for Matrix-Assisted Laser Desorption/Ionization-Imaging Mass Spectrometry Analysis
Summary
This protocol aimed to describe detailed guidance on the preparation of hard seed sample sections with low water content for MALDI-IMS analysis, maintaining analytes’ original distribution and abundance and providing high-quality signal and spatial resolution.
Abstract
Matrix-assisted laser desorption/ionization-imaging mass spectrometry (MALDI-IMS) is applied to identify compounds in their native environments. Currently, MALDI-IMS is frequently used in clinical analysis. Still, an excellent perspective exists for better applying this technique to understand chemical compounds' physiological information in plant tissues. However, preparation may be challenging for specific samples from botanical materials, as MALDI-IMS requires thin slices (12-20 µm) for appropriate data acquisition and successful analysis. In this sense, previously, we developed a sample preparation protocol to obtain thin sections of Euterpe oleracea (açaí palm) hard seeds, enabling their molecular mapping by MALDI-IMS.
Here, we show that the developed protocol is suitable for preparing other seeds from the same genus. Briefly, the protocol was based on submerging the seeds in deionized water for 24 h, embedding samples with gelatin, and sectioning them in an acclimatized cryostat. Then, for matrix deposition, an xy motion platform was coupled to an electrospray ionization (ESI) needle spray using a 1:1 (v/v) 2,5-dihydroxybenzoic acid (DHB) and methanol solution with 0.1% trifluoroacetic acid at 30 mg/mL. E. precatoria and E. edulis seed data were processed using software to map their metabolite patterns.
Hexose oligomers were mapped within sample slices to prove the adequacy of the protocol for those samples, as it is known that those seeds contain large amounts of mannan, a polymer of the hexose mannose. As a result, peaks of hexose oligomers, represented by [M + K]+ adducts of (Δ = 162 Da), were identified. Thus, the sample preparation protocol, previously developed tailor-made for E. oleracea seeds, also enabled MALDI-IMS analysis of two other hard palm seeds. In short, the method could constitute a valuable tool for research in the morpho-anatomy and physiology of botanical materials, especially from cut-resistant samples.
Introduction
Matrix-assisted laser desorption/ionization-imaging mass spectrometry (MALDI-IMS) is a powerful method that allows two-dimensional biomolecule assignment, provides untargeted investigation of ionizable compounds, and determines their spatial distribution, especially in biological samples1,2. For two decades, this technique has enabled the simultaneous detection and identification of lipids, peptides, carbohydrates, proteins, other metabolites, and synthetic molecules such as therapeutic drugs3,4. MALDI-IMS facilitates chemical analysis in a tissue sample surface without extraction, purification, separation, labeling, or staining agents of biological samples. However, for successful analysis, a pivotal step in this technique is the sample preparation, particularly in plant tissues, which are specialized and modified to widespread complex organs due to environmental acclimatization5.
Because of the inherent plant tissue physicochemical properties, there is a need for an adapted protocol to suit the requirements of MALDI-IMS analysis and preserve the tissue's original shape during sectioning preparation6,7. In the case of unconventional samples, such as seeds, established protocols8 are not applicable because these tissues have rigid cell walls and low water content, which can easily cause section fragmentation and lead to compound delocalization9.
Our research group has published experimental data on molecular mapping and an adapted protocol for MALDI-IMS analysis of açaí (Euterpe oleracea Mart.) seed10,11,12, which is a byproduct generated in high amounts during the production of the rentable açaí pulp13. The idea was to develop a protocol for in situ mapping of different metabolites in açaí seeds, helping to suggest possible uses for this agricultural waste that are currently not being explored commercially. However, due to the resistance of the açaí seed, it was necessary to tailor-make a protocol to obtain proper sample sectioning from MALDI-IMS analysis.
In this context, the economically important açaí pulp has motivated the increasing commercialization of other fruits from Euterpe genus palm trees with similar sensory characteristics. The two emerging palm trees' fruits that have been produced on an industrial scale as an alternative to açaí14,15 are E. precatoria (known as açaí-do-amazonas), which grows in the Amazon dryland, and E. edulis (known as juçara), which is typical from the Atlantic Forest. Nevertheless, the consumption of açaí-do-amazonas and juçara leads to the same accumulation of resistant and inedible seeds that are not availed and have not been studied so far regarding their detailed chemical composition.
Thus, we demonstrate here that the previously devised protocol can be used, with few adaptations, to analyze E. precatoria and E. edulis seeds for molecular mapping by MALDI-IMS, proving to be a powerful tool that can be used for analysis of the composition of these resources and can help to determine their potential biotechnological uses. Moreover, the detailed description provided here can aid others with similar difficulties in preparing resistant materials for MALDI-IMS analysis.
Protocol
Euterpe precatoria seeds were kindly donated by the Instituto Nacional de Pesquisas da Amazônia (Manaus, Brazil), and Euterpe edulis seeds were kindly donated by the Silo – Arte e Latitude Rural (Resende, Brazil) after the industrial depulping process. The seeds were maintained in sealed plastic boxes at room temperature.
1. Matrix-assisted laser desorption/ionization-imaging mass spectroscopy (MALDI-IMS)
- Seed sectioning protocol
- Allow three seeds from each species to sit in deionized water for 24 h.
- The next day, turn the cryostat (see Table of Materials) on and let it reach -20 °C.
- Take the wet seeds out of the water. Cut the seeds in half using a microtome blade (see Table of Materials).
- Prepare a fresh, warm (10%) gelatin solution (see Table of Materials).
- Place half of the seed on a mold and fill it with fresh-made gelatin. Freeze at -80 °C for 2 h before taking to the cryostat.
- Attach the embedded seed to the cryostat support using an optimal cutting temperature compound (OCT, see Table of Materials) and leave for 10 min inside the cryostat for OCT hardening.
- Add copper double-faced adhesive tape (see Table of Materials) to an indium tin oxide-coated glass slide (ITO slide; see Table of Materials).
- Produce 20 µm thick sections from each species and place them on the copper double-faced adhesive tape adhered to the ITO glass slide.
NOTE: At this point, the slices collected on the slides can be stored in a -80 °C freezer; alternatively, proceed to matrix deposition. The slides must be placed in a holder slide box, flushed with gaseous N2, and sealed with film to prevent sample oxidation (see Table of Materials).
- Matrix deposition
- Place the slide containing slices in a vacuum desiccator until it reaches room temperature.
- Make teaching marks using a correction pen in each slide corner. Scan the slide using a table scanner. Set to the resolution of 4,800 ppi.
- Use an analytical balance to weigh 30 mg of 2,5-dihydroxybenzoic acid (DHB; see Table of Materials) and prepare 1 mL of 1:1 methanol:0.1% trifluoroacetic acid (TFA; Table of Materials) solution to dissolve the DHB.
- Fill a 1 mL glass syringe (see Table of Materials) with DHB solution and place it in a syringe pump (see Table of Materials) set to a flow rate of 0.8 mL/h.
- Using PEEK tubing, connect the syringe to an atmospheric pressure chemical ionization (APCI) needle (see Table of Materials).
- Connect N2 to the APCI needle and set it to a 12.5 psi flow rate.
- Attach the APCI needle to the xy motion platform (see Table of Materials). Ensure that the tip of the APCI needle is 4 cm above the slide.
- Using the drawing software (see Table of Materials and Supplementary Figure 1), set the xy motion platform to follow the template. The template consists of horizontal parallel lines spaced by 1 mm.
- To achieve matrix deposition, wait for the xy motion platform to repeat the template 20 times.
CAUTION: Matrix deposition must be carried out in a chemical fume hood.
- Imaging acquisition
- Place the slide in the mass spectrometer (see Table of Materials).
- Use correction pen marks to set teaching points on the referenced software (see Table of Materials).
- Set the laser power (60%), laser focus (medium), number of shots (100), and polarity in the software (see Table of Materials). Set 99% data reduction factor and save the FID file for posterior data calibration. Save the method.
- Delimitate the area to be analyzed using the add polygon measurement region tool from the mass spectrometer software. Edit the measurement region parameters indicating the method saved in the previous step and the raster width to 100 µm (Supplementary Figure S4A).
- Start imaging acquisition.
- Data analysis
- Use matrix cluster and known contaminants to create a mass list in the referenced software (see Table of Materials) in the calibrant tab (Supplementary Figure S4B).
- Open the data to be calibrated in the referenced software (see Table of Materials). In the calibration tab, open the mass list created and open a dialog box by right-clicking and choose the set lock masses option (Supplementary Figure S4C).
- Select Gaussian window mode with 0.5 Gaussian broadening and 3.5 Line broadening. Leave online calibration unchecked. Select mode (single), threshold (1,000), and mass tolerance (5 ppm). Calibrate data with process and save the 2D data tool (Supplementary Figure S4C).
- After calibration, export the data to SCiLS lab or other compatible software and set the desired m/z value threshold (range chosen: 150 to 2,500).
NOTE: Depending on the file size or the computer's characteristics, this might take some time. - Choose a normalization method between total ion count (TIC) or root mean square (RMS).
NOTE: For this analysis, TIC was chosen. - If the analytes to be mapped are known, plot each m/z value for each analyte and save the generated images and the spectral average plot.
NOTE: In this work, m/z values from hexose oligomers were chosen by considering the potassium adduct.
- Use matrix cluster and known contaminants to create a mass list in the referenced software (see Table of Materials) in the calibrant tab (Supplementary Figure S4B).
2. Energy-dispersive spectroscopy (EDS)
- Seed sectioning protocol
- Acquire a thin seed slice in a sectioning saw machine (see Table of Materials).
- Cut the seeds with the following parameters: 500 RPM cutting speed, 100 N load charge, and 15 HC Diamond Wafering Blade (see Table of Materials).
NOTE: Handle the manual removal of fibers from the seeds due to the necessary adhesion to the hold in the precision vises attached to the goniometer. Clean the blade before use, cutting a section of the seed to prevent adding undesirable minerals during the analysis. - Clear the samples with compressed air to remove all the particle residues from the cut (see Table of Materials).
- Fix a seed section in carbon double-sided conductive tape (see Table of Materials) in the support.
- Analysis conditions
- Attach the support with a seed section inside the vacuum chamber of a scanning electron microscope (SEM) coupled with EDS (see Table of Materials).
- Acquire electron micrographs on a model microscope with 20 kV acceleration and a spot size of 4.0, using secondary electron signals (see Table of Materials), backscattered electrons (solid state detector fixed on the polar piece), and mixtures of these two types of signals (MIX), building artificially colored images.
- For the acquisition of EDS spectra, use a spectrometer (see Table of Materials) coupled with the aforementioned SEM. The condition configuration of the image acquisition from EDS is the same as the SEM.
- Set tilt degrees, elevation, and azimuth at 0.0, 35.0, and 0.0, respectively.
- Data acquisition
- Set three different areas with 91x magnification of the seed section to acquire data.
- Identify the peaks in the spectrum upon termination of acquisition or during acquisition. Use the Confirm Elements step button to identify the peaks manually.
- Set the acquisition time to 60 s for each selected area. Set the process time to five; the parameters are available for a process time of one to six.
NOTE: The signs of the elements are constant and add up, while the noises are random and deleterious. The longer the processing time, the lower the noise. - The default settings to display significant quantitative results are above two sigmas (standard deviation). Set two sigmas to zero to reduce undesirable results.
- Normalize each intensity of the elements; this is a standardized parameter in the software (see Table of Materials).
- To analyze the data, export it in a DOC file.
- Data analysis
- Ensure the accurate measurement of peak intensities for quantitative elemental analysis.
- Overlapping peaks require deconvolution for better peak separation; subtract a noisy background when required.
- When there is no overlapping, increase the gain to amplify the signals and improve the spectrum quality.
Representative Results
The devised protocol enabled MALDI-IMS analysis of E. precatoria and E. edulis seeds. As a result, we could confirm carbohydrates' molecular weight and degree of polymerization (DP) as a partial structural elucidation. The molecular information provided by the MALDI-IMS analysis (Figure 1 and Figure 2) exhibited peaks representing [M+K]+ adducts of hexose oligomers (Δ = 162 Da) without adding salt to the matrix. Hexose dimers (m/z 381), trimers (m/z 543), tetramers (m/z 705), pentamers (m/z 867), hexamers (m/z 1029), and up to 14-unit oligomers (m/z 2325) were identified in both seed tissues. The box plots for both samples (Figure 3) indicate the peak intensity of each hexose oligomer found in the seed endosperm, demonstrating their distributions and a slightly higher content of high DP oligomers.
Previously, we provided EDS data on E. oleracea seeds, which indicated that potassium adducts detected in the MALDI-IMS analysis were due to the sample's intrinsic characteristic of elevated potassium content10. This study also analyzed E. precatoria and E. edulis seeds to identify the main elements in the sample's sections (Supplementary Figure S2 and Supplementary Figure S3). The atomic composition observed on the tissue surface was homogeneously distributed, showing mainly nonmineral elements, such as carbon and oxygen, and lower mineral content. In both samples, potassium was the main mineral element found by EDS analysis (Supplementary Figure S2 and Supplementary Figure S3).
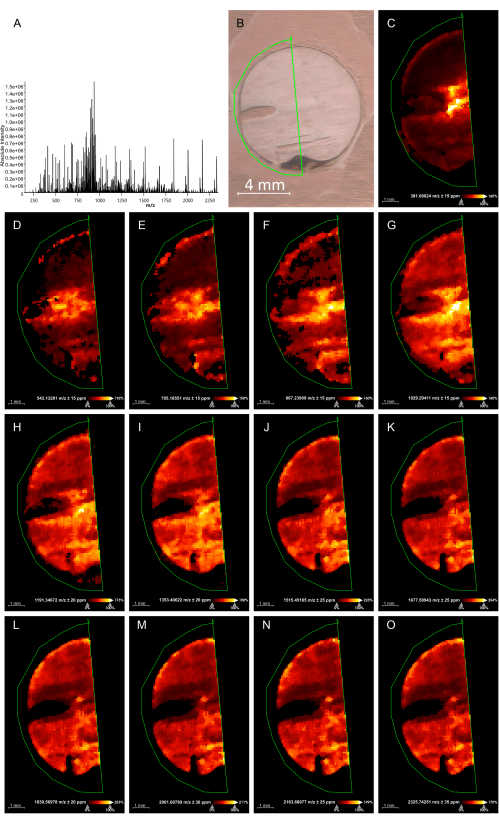
Figure 1: Hexose oligomer distribution in Euterpe precatoria seeds by MALDI-IMS in positive mode. (A) Mass spectrum obtained. (B) Histological image and the frame used for the MALDI-IMS analysis. (C–O) Image representations of a specific m/z signal describing its intensity in the tissue for each [M+K]+ adduct from hexose dimer (C), trimer (D), up to 14 units (E–O) in the seeds' endosperm. Scale bar = 4 mm (B). Abbreviation: MALDI-IMS = matrix-assisted laser desorption/ionization-imaging mass spectrometry. Please click here to view a larger version of this figure.
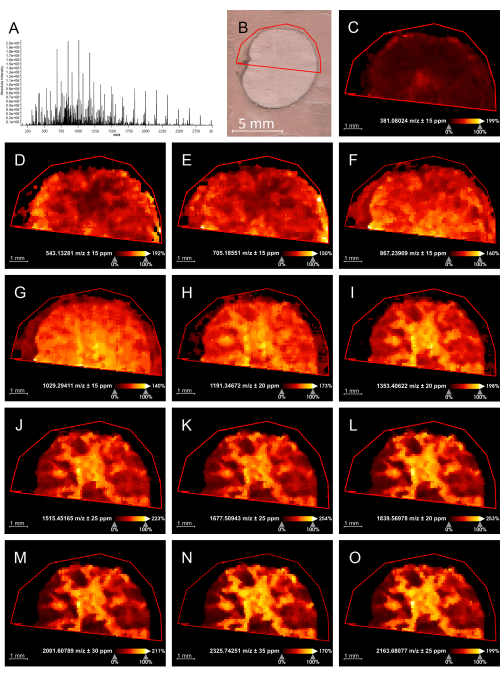
Figure 2: Hexose oligomer distribution in Euterpe edulis seeds by MALDI-IMS in positive mode. (A) Mass spectrum obtained. (B) Histological image and the frame used for the MALDI-IMS analysis. (C–O) Image representations of a specific m/z signal describing its intensity in the tissue for each [M+K]+ adduct from hexose dimer (C), trimer (D), up to 14 units (E–O) in the seeds' endosperm. Scale bar = 5 mm (B). Abbreviation: MALDI-IMS = matrix-assisted laser desorption/ionization-imaging mass spectrometry. Please click here to view a larger version of this figure.
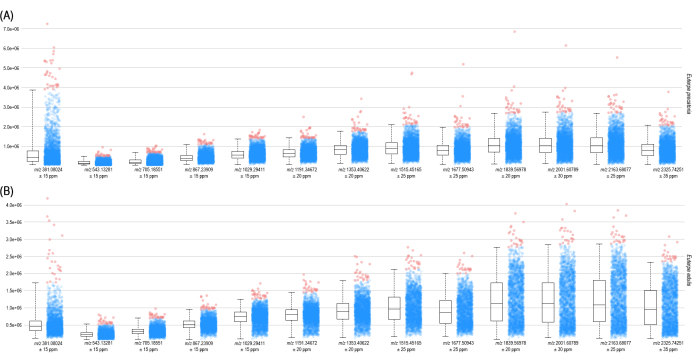
Figure 3: Box plot visualizations. (A) E. precatoria and (B) E. edulis, indicating the peak intensity and distributions of each hexose oligomer found in the seed tissue. Please click here to view a larger version of this figure.
Supplementary Figure S1: Matrix coating template. The DHB matrix was sprayed onto the platform using drawing software for equal distribution on a 75 mm x 25 mm slide of horizontal parallel lines spaced by 1 mm. Please click here to download this File.
Supplementary Figure S2: Elementary composition analysis of E. precatoria seeds by energy dispersive X-ray spectrometry. (A) E. precatoria seed sample surface section. (B) Major elements semi-quantified. The main components correspond to about 47.0% carbon (C), 52.7% oxygen (O), and 0.2% potassium (K). Please click here to download this File.
Supplementary Figure S3: Elementary composition analysis of E. edulis seeds by energy dispersive X-ray spectrometry. (A) E. edulis sample surface section. (B) Major elements semi-quantified. The main components correspond to about 47.7% carbon (C), 53.8% oxygen (O), 0.3% potassium (K), and some traces of chlorine (Cl). Please click here to download this File.
Supplementary Figure S4: Delimitation of the area in an image and data analysis. (A) Delimitation of the area to be analyzed and editing the parameters and raster width to 100 µm. (B) Identification of a matrix cluster and creation of a mass list. (C) Calibration of data and saving the 2D data tool. Please click here to download this File.
Discussion
Plants are composed of specialized tissues for specific biochemical functions. Therefore, the sample preparation protocol for MALDI-IMS must be designed according to various plant tissues with specific physicochemical properties, as samples must maintain their original analyte distribution and abundance for high-quality signal and spatial resolution8.
Prior to MALDI-IMS analysis, the primary consideration is collecting and storing samples properly. However, in plants, sample preparation often varies depending on the analyzed tissue. For example, cell wall lignification and water content in plant tissues may pose challenges during sectioning to maintain an accurate representation of the morphology in the sample7.
MALDI-IMS analysis can be divided into a workflow consisting of tissue preparation and sectioning, matrix coating, and data analysis. However, the quality and authenticity of the imaging results are directly affected by the sample preparation method, which is a crucial step. The sample preparation method used in this study was based on a previously published protocol for E. oleracea mature seed10. To validate the sectioning protocol for other samples, we evaluated the steps for sample sectioning using E. precatoria and E. edulis seeds to acquire thin (20 µm) sections and enable MALDI-IMS analysis with proper resolution. Most information is described in detail in the protocol section; however, we emphasize that manual external fiber layer removal is an important step to soften the seed, allowing blade sectioning.
Standard histological workflows are usually discouraged for MALDI-IMS, since fixation procedures may lead to ion suppression effects. Cryosectioning is the most commonly used method for sample preparation. However, sample shrinkage or crumbling may occur, providing metabolite dislocation and hindering the biological interpretation5,8. Another possibility for soft tissues such as leaves and flowers is the imprinting method. However, for this study, because of the hard tissue nature and low water content in the samples, the technique used before sectioning was to embed the sample with specific material to provide accurate, high-quality tissue sections.
It is noteworthy that tissue thickness could lead to a reduced signal intensity. Overall, MALDI-IMS requires a <20 µm slice thickness to provide adequate resolution. However, hard and large plant tissues generally fracture during a thin sample's sectioning. To solve this problem, the protocol used a conductive adhesive tape made of copper16, reducing the sections' distortion and facilitating attachment to the slide. Furthermore, obtaining a thin section of the whole seed is difficult without soaking it in deionized water overnight. However, embedding the sample for this amount of time could cause metabolite dislocation; therefore, in a previously published study with E. oleracea seed11, we compared small sections (wet and dry) with and without the soaking step, from which the results indicated no dislocation for the procyanidins found in the seeds tegument.
Matrix deposition is another critical step for matrix homogeneity on the tissue section surface, ensuring analyte ionization. For the MALDI matrix deposition, we evaluated, in preliminary tests, different matrices, such as 9-aminoacridine (9AA), α-cyano-4-hydroxycinnamic acid (HCCA), 1,5-diaminonapthalene (DAN), and 2,5-dihydroxybenzoic acid (DHB). DHB was chosen as the matrix since it provided better resolution on the carbohydrate signals (data not shown) and also due to its "universal analysis" nature1; however, the original method employed a sublimation approach10,17, whereas this time we utilized automatic robotic spraying to control the matrix coating conditions and provide a more uniform application and smaller matrix crystals for a higher-resolution image and to avoid ion suppression effects.
E. precatoria and E. edulis display globose-shaped seeds with voluminous homogeneous endosperm covered by a thin external tegument, which only differs from E. oleracea seeds because of its characteristic ruminated endosperm10,18,19. The unconventional natures found for these plant samples provided similar processing difficulties for the adapted sectioning and matrix deposition protocols. However, these results indicate that the sample preparation was adequate and provided reliable and effective images, which determined the spatial localization of metabolites in E. precatoria and E. edulis seeds. The MALDI-IMS analyses enabled the potential exploitation of these raw materials for the first time in the literature based on their molecular mapping data.
The EDS data indicated a presence of potassium lower than 1% in the analyzed seed tissues of E. edulis and E. precatoria. However, the concentration was high enough to provide [M+K]+ peaks during the MALDI-IMS analysis. Previously published studies had identified potassium as the main mineral found in both seeds20,21, indicating a natural presence of potassium in Euterpe genus seeds and explaining the adducts in the MALDI-IMS data of these species.
In this study, we also evaluated the proof of concept that the protocol devised for the E. oleracea seed was implemented for E. precatoria and E. edulis seeds. The reserve polysaccharide found in the açaí (E. oleracea) seed's endosperm is majorly mannan22, whose organization is highly crystalline, enhancing endosperm hardness to obtain thin sections of mature seeds10,19. Previous studies quantified the carbohydrates in the endosperm of both seeds by gravimetric methods, finding a content above 90% of the seeds' dry weight20,21. Moreover, a histochemistry study in E. edulis suggested the presence of mannan in the endosperm through its highly crystalline and strong birefringence under polarized light18. Nevertheless, there are no data on the chemical composition of the reserve carbohydrates in both seeds' endosperm. Because of their phylogenetic proximity to E. oleracea seed, the hexose oligomers identified by MALDI-IMS are also expected to be mannan-oligosaccharides. Still, there is a need for future studies that evaluate the chemical composition of these reserve polysaccharides to confirm the mannan and mannan-oligosaccharide structures in E. precatoria and E. edulis seeds.
This protocol can be applied as a useful tool in studies of those seeds, which go beyond our described goal here. For example, this technique could benefit the analysis of biochemical processes during seed development and germination. Finally, the detailed description provided here can be a helpful starting point for devising adapted protocols for analysis by MALDI-IMS of other resistant materials from plants.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was financed by Serrapilheira Institute (Serra-1708-15009), and Carlos Chagas Filho Foundation for Supporting Research in the State of Rio de Janeiro (FAPERJ-JCNE-SEI-260003/004754/2021). Serrapilheira Institute and the National Council for Scientific and Technological Development (CNPq) granted scholarships for Dr. Felipe Lopes Brum and Dr. Gabriel R. Martins (Institutional Capacity Building Program/INT/MCTI). The Coordination for the Improvement of Higher Education Personnel (CAPES) is acknowledged for granting a Master's scholarship for Mr. Davi M. M. C. da Silva. Centro de Espectrometria de Massas de Biomoléculas (CEMBIO-UFRJ) is recognized for the services provided with MALDI-IMS analyses, and Mr. Alan Menezes do Nascimento and the Centro de Caracterização em Nanotecnologia para Materiais e Catálise (CENANO-INT), funded by MCTI/SISNANO/INT-CENANO-CNPQ grant Nº 442604/2019, are thanked for the elementary composition analysis.
Materials
| 1 mL Gastight Syringe Model 1001 TLL, PTFE Luer Lock | Hamilton Company | 81320 | |
| 2,5-Dihydroxybenzoic acid | Sigma Aldrich Co, MO, USA | 149357 | |
| APCI needle | Bruker Daltonik, Bremen, Germany | 602193 | |
| AxiDraw V3 xy motion platform | Evil Mad Scientist, CA, USA | 2510 | |
| Carbon double-sided conductive tape | |||
| Compass Data Analysis software | creation of mass list | ||
| Compressed air | |||
| copper double-faced adhesive tape | 3M, USA | 1182-3/4"X18YD | |
| Cryostat CM 1860 UV | Leica Biosystems, Nussloch, Germany | ||
| Diamond Wafering Blade 15 HC | |||
| Everhart-Thornley detector | |||
| FlexImaging | Bruker Daltonik, Bremen, Germany | image acquisition | |
| FTMS Processing | Bruker Daltonik, Bremen, Germany | data calibration | |
| Gelatin from bovine skin | Sigma Aldrich Co, MO, USA | G9391 | |
| High Profile Microtome Blades Leica 818 | Leica Biosystems, Nussloch, Germany | 0358 38926 | |
| indium tin oxide coated glass slide | Bruker Daltonik, Bremen, Germany | 8237001 | |
| Inkscape | Inkscape Project c/o Software Freedom Conservancy, NY, USA | ||
| IsoMet 1000 precision cutter | Buehler, Illinois, USA | ||
| Methanol | J.T.Baker | 9093-03 | |
| Mili-Q water | 18.2 MΩ.cm | ||
| Oil vacuum pump | |||
| Optimal Cutting Temperature Compound | Fisher HealthCare, Texas, USA | 4585 | |
| Parafilm "M" Sealing Film | Amcor | HS234526B | |
| Quanta 450 FEG | FEI Co, Hillsboro, OR, USA | ||
| SCiLS Lab (Multi-vendor support) MS Software | Bruker Daltonik, Bremen, Germany | ||
| Software INCA Suite 4.14 V | Oxford Instruments, Ableton, UK | ||
| Solarix 7T | Bruker Daltonik, Bremen, Germany | ||
| Syringe pump | kdScientific, MA, USA | 78-9100K | |
| Trifluoroacetic acid | Sigma Aldrich Co, MO, USA | 302031 | |
| X-Max spectrometer | Oxford Instruments, Ableton, UK |
References
- Buchberger, A. R., DeLaney, K., Johnson, J., Li, L. Mass spectrometry imaging: a review of emerging advancements and future insights. Analytical Chemistry. 90 (1), 240-265 (2018).
- Heeren, R. M. A. MALDITechniques in Mass Spectrometry Imaging. Encyclopedia of Spectroscopy and Spectrometry. , (2017).
- Shariatgorji, M., Svenningsson, P., Andrén, P. E. Mass spectrometry imaging, an emerging technology in neuropsychopharmacology. Neuropsychopharmacology. 39 (1), 34-49 (2014).
- Zaima, N., Hayasaka, T., Goto-Inoue, N., Setou, M. Matrix-assisted laser desorption/ionization imaging mass spectrometry. International Journal of Molecular Sciences. 11 (12), 5040-5055 (2010).
- Qin, L., et al. Recent advances in matrix-assisted laser desorption/ionisation mass spectrometry imaging (MALDI-MSI) for in Situ analysis of endogenous molecules in plants. Phytochemical Analysis. 29 (4), 351-364 (2018).
- Bhandari, D. R., et al. High resolution mass spectrometry imaging of plant tissues: Towards a plant metabolite atlas. Analyst. 140 (22), 7696-7709 (2015).
- Boughton, B. A., Thinagaran, D., Sarabia, D., Bacic, A., Roessner, U. Mass spectrometry imaging for plant biology: a review. Phytochemistry Reviews. 15 (3), 445-488 (2016).
- Dong, Y., et al. Sample preparation for mass spectrometry imaging of plant tissues: a review. Frontiers in Plant Science. 7, 60 (2016).
- Zhang, Y. X., Zhang, Y., Shi, Y. P. A reliable and effective sample preparation protocol of MALDI-TOF-MSI for lipids imaging analysis in hard and dry cereals. Food Chemistry. 398, 133911 (2023).
- Brum, F. L., Martins, G. R., Mohana-Borges, R., da Silva, A. S. The acquisition of thin sections of açaí (Euterpe oleracea Mart.) seed with elevated potassium content for molecular mapping by mass spectrometry imaging. Rapid Communications in Mass Spectrometry. , e9474 (2023).
- Martins, G. R., et al. Chemical characterization, antioxidant and antimicrobial activities of açaí seed (Euterpe oleracea Mart.) extracts containing A- and B-type procyanidins. LWT. 132, 109830 (2020).
- Martins, G. R., et al. Phenolic profile and antioxidant properties in extracts of developing açaí (Euterpe oleracea Mart.) seeds. Journal of Agricultural and Food Chemistry. 70 (51), 16218-16228 (2022).
- Jorge, F. T. A., Silva, A. S. A., Brigagão, G. V. Açaí waste valorization via mannose and polyphenols production: techno-economic and environmental assessment. Biomass Conversion and Biorefinery. , (2022).
- Carvalho, L. M. J., Esmerino, A. A., Carvalho, J. L. V. Jussaí (Euterpe edulis): a review. Food Science and Technology. 42, (2022).
- Yamaguchi, K. K. d. L., Pereira, L. F. R., Lamarão, C. V., Lima, E. S., Veiga-Junior, V. F. d. Amazon acai: chemistry and biological activities: A Review. Food Chemistry. 179, 137-151 (2015).
- Wu, R., et al. Copper adhesive tape attached to the reverse side of a non-conductive glass slide to achieve protein MALDI-imaging in FFPE-tissue sections. Chemical Communications. 57 (82), 10707-10710 (2021).
- Dufresne, M., Patterson, N. H., Norris, J. L., Caprioli, R. M. Combining salt doping and matrix sublimation for high spatial resolution MALDI imaging mass spectrometry of neutral lipids. Analytical Chemistry. 91 (20), 12928-12934 (2019).
- Aguiar, M. O., de Mendonça, M. S. Morfo-anatomia da semente de Euterpe precatoria Mart (Palmae). Revista Brasileira de Sementes. 25, 37-42 (2003).
- Panza, V., Láinez, V., Maldonado, S. Seed structure and histochemistry in the palm Euterpe edulis. Botanical Journal of the Linnean Society. 145 (4), 445-453 (2004).
- Alves, V. M., et al. Provenient residues from industrial processing of açaí berries (Euterpe precatoria Mart): nutritional and antinutritional contents, phenolic profile, and pigments. Food Science and Technology. 42, (2022).
- Inada, K. O. P., et al. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. Journal of FunctionalFoods. 17, 422-433 (2015).
- Monteiro, A. F., Miguez, I. S., Silva, J. P. R. B., Silva, A. S. High concentration and yield production of mannose from açaí (Euterpe oleracea Mart.) seeds via mannanase-catalyzed hydrolysis. Scientific Reports. 9 (1), 10939 (2019).

