Abstract
Source: de Vries, S. E., Clandinin, T. Optogenetic Stimulation of Escape Behavior in Drosophila melanogaster. J. Vis. Exp. (2013).
Optogenetics enables the use of light to manipulate neurons that are genetically engineered to express specific opsins–light-sensitive proteins whose light activation triggers a change in the state of the neuron. Here we describe an optogenetic approach in Drosophila using the opsin Channelrhodopsin2. The example protocol features an optogenetics assay set to study the neuronal circuitry behind the fly's escape behavior.
Protocol
This protocol is an excerpt from de Vries and Clandinin, Optogenetic Stimulation of Escape Behavior in Drosophila melanogaster, J. Vis. Exp. (2013).
1. Generate Channelrhodopsin Flies
- Cross UAS-ChR2 flies with the Gal4 driver of your choosing, we use G105-Gal4, which is expressed in Foma-1 neurons in the optic lobe.
- To eliminate the possibility of a visual response to the blue light stimulation, both fly lines are in a w+norpA background.
- End result: w+norpA;G105-Gal4/UAS-ChR2 +
- After adult flies eclose, put selected females on fresh food, supplemented with 10 μM all-trans-retinal (a co-factor required for ChR2) and protected from light, for 3 days before performing the behavioral assay.
2. Make 10 μM All-trans-retinal Enhanced Food
- Dissolve 100 mg of all-trans-retinal in 17.6 ml of 95% ethanol to make 20 mM retinal. Keep all-trans-retinal protected from light at all times.
- Melt standard cornmeal fly food in microwave, and let cool until warm to touch.
- Mix 50 μl of 20 mM all-trans-retinal into vials of 10 ml of fly food.
- Let vials cool and keep protected from light.
3. Equipment
- Pipet tips: Standard 1,000 μl pipet tips are cut near the tip, creating a pore diameter of ~2.25 mm.
- Platform (see Figure 1).
- A Delrin base, 17 cm X 25 cm, was constructed with threaded holes at each corner to fit ¼" NPT coolant hose connectors.
- A vertical holder, made from Delrin, is attached to the center of the base. The overall dimensions are 25 mm X 40 mm X 65 mm (width X depth X height). A 10 mm wide groove runs the length of the holder, with a thumb screw at the bottom. A platform is attached to the top of the holder, 25 mm X 40 mm X 10 mm, with a 3.5 mm diameter hole aligned with the groove in the holder.
- LED arrays (see Figure 1).
- Four arms of coolant hose, ~18 cm long, are affixed to the platform base using the coolant hose connector. Coolant hose is used only as structural support and is not used for cooling purposes.
- Properly spaced grooves are cut into the final piece of coolant hosing of each arm to affix a heat sink to the end of each arm.
- A blue LED Rebel Tri-Stars is mounted to each heat sink using pre-cut thermal adhesive tape. A Carclo 18° Tri-lens is affixed to each Tri-Star.
- LED Tri-Stars are wired to the BuckPuck DC drivers and a power supply as specified. We have arranged our set-up with each BuckPuck powering two Tri-Stars in series.
- Illumination of all four LED Tri-Stars at 700 mA yielded an irradiance of 713 W/m2 on our platform.
- Camera: The camera is mounted on a small tripod and focused on the top of the platform.
4. Behavioral Assay
- Briefly anaesthetize flies on ice.
- Place individual flies in pipet tips, using tape to close both ends of the tip.
- After the flies have awoken and are actively exploring the pipet tip, remove the tape and place a pipet in the groove in vertical holder. The thumbscrew is used to secure the pipet tip in place and to close the bottom of the tip.
- As the fly explores the pipet tip (typically 30 – 60 sec), start the camera recording just before the fly emerges from the tip onto the platform.
- After the fly has emerged onto the platform, wait 1-2 seconds, and turn on the blue LEDs. Use a timer to manually measure the time until the fly initiates flight.
Representative Results
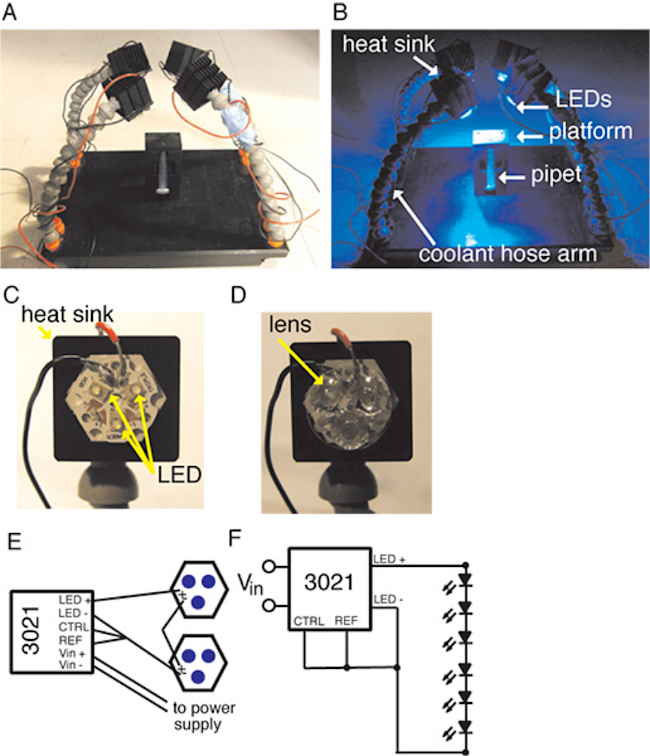
Figure 1: Experimental set-up showing the platform with the vertical holder and the four coolant hosing arms holding heat sinks with LED arrays affixed to them. (A) The set-up under ambient illumination. (B) The set-up when the LEDs are illuminated. (C) A close up view of a Tri-Star LED on the heat sink. (D) A close up of the Tri-Star with the tri-lens attached. (E) A schematic of the Buckpuck Driver and LED circuit. (F) Circuit diagram for the BuckPuck and LED circuit. Please click here to view a larger version of this figure.
Materials
| Reagent | |||
| All-trans Retinal | Advance Scientific Chemical Inc | R3041 | |
| Equipment | |||
| Heat Sink 9.2 C/W | Luxeonstar | LPD30-30B | 30 mm square X 30 mm high |
| Carclo 18° Tri-Lens | Luxeonstar | 10507 | |
| Blue Rebel LED on Tri-Star Base | Luxeonstar | MR-B0030-20T | 470 nm, 174 lm @ 700 mA. |
| 700 mA BuckPuck DC Driver | Luxeonstar | 3021-D-E-700 | |
| Wiring Harness for BuckPuck Driver | Luxeonstar | 3021-HE | |
| Pre-cut thermal adhesive tape | Luxeonstar | LXT-S-12 | 20 mm Hex Base |
| Snap-Loc Coolant Hose, ¼" ID | McMaster-Carr | 5307K49 | |
| Snap-Loc Coolant Hose Connector | McMaster-Carr | 5307K39 | ¼" NPT Male |
| Laboratory Grade Switching Mode Programmable DC Power Supply | BK Precision | 1698 | |
| Exilim camera | Casio | EX-FH20 |

