Layered Agar Mounting: Preparing Live Zebrafish Embryos for Long-Term Imaging with an Inverted Microscope
Abstract
Source: Upadhyay S., et.al. A Layered Mounting Method for Extended Time-Lapse Confocal Microscopy of Whole Zebrafish Embryos. J. Vis. Exp. (2020).
This article describes a method to mount live zebrafish embryos for long term imaging. This method is cost effective and easy to perform using regular glass-bottom microscopy dishes for imaging on Inverted Microscope. The mounting is performed in layers of agarose at different concentrations.
Protocol
1. Preparation of embryos
- After mating, harvest embryos in E3 in a Petri dish and incubate them at 26.5 °C for about 28 h before mounting.
NOTE: This slows down the development of the embryos so that the embryos are approximately at 30 somite stage at the beginning of imaging. - Anesthetize embryos in 0.016-0.020% Tricaine in E3. To inhibit pigmentation, add PTU to a concentration of 200 µM.
- Dechorionate the embryos using forceps under a dissecting microscope. Using two forceps, grip and gently pull the chorion apart to release the embryo.
2. Mounting in agarose
NOTE: The developed mounting method requires two different concentrations of low-melt agarose in E3 with 0.02% Tricaine and PTU as needed. The first agarose solution contains an optimal concentration of agarose at which the distortion and motility are at a minimum. The optimization is described in step 5 below.
- Heat the agarose solutions for the two layers (concentration defined in step 5 below and 1%) to 65 °C. Let the agarose cool down to approximately 30 °C just before mounting so that the embryo is not harmed by the heat. For mounting, use 35 mm glass bottom dishes with a No. 0 cover glass bottom. The cover glass attached to the bottom of the dish creates a 10 mm shallow (approx. 1.2 mm deep) well, in which the embryo is to be placed.
NOTE: In this case, the concentration with the least motility and distortions were between 0.025 to 0.040% agarose. - Gently place a dechorionated embryo with one of its lateral sides toward the bottom of the dish using a glass pipette or micropipette. If using a micropipette, cut the outer part of the tip to increase the size of the opening to fit the embryo (Figure 1A). Carefully remove any remaining E3 with a micropipette.
- Add the first agarose solution to the small well created by the cover glass attached to the bottom of the dish to cover the embryo (Layer 1) (Figure 1B). Ensure that the agarose covers the small well but will not overflow it.
- Cover the small well with a cover glass (22 mm x 22 mm) (Figure 1C) to create a narrow agarose filled space with the embryo between the two cover glasses.
- Place a layer of 1% agarose solution on top of the cover glass all over the bottom of the dish (Layer 2) (Figure 1D). As this layer solidifies, it holds the cover glass in place.
- Fill the remaining portion of the dish with E3 containing 0.02% Tricaine to keep the system hydrated (Layer 3) (Figure 1E).
NOTE: In this setup, the cover glass and 1% agarose protect the bottom layer from getting diluted.
3. Optimization of agarose solution for layer 1
- To identify the optimal concentration of agarose for Layer 1, use a multiscale grid search approach. Mount embryos in increasing concentrations of agarose ranging from 0.01% to 1% followed by time-lapse imaging of embryo growth restriction and motility in the field of view. Identify the concentrations where both the distortion and motility are at a minimum.
- To optimize the concentration of agarose further, mount the embryos using a finer range of concentrations of agarose (e.g., between 0.025 and 0.040% agarose) depending on the concentration found to be best in step 3.1 (e.g., 0.025%, 0.028%, 0.031%, etc.).
NOTE: In our laboratory, the optimal agarose concentration was around 0.03%.
Representative Results
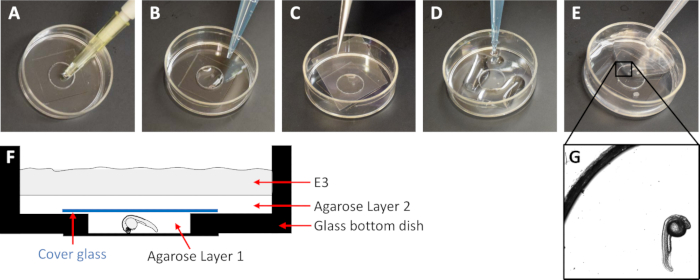
Figure 1 : Description of mounting method. (A) Add the zebrafish embryo to the small well created by the glass bottom in the 35 mm dish. (B) Add agarose layer 1 to the small well to cover the embryo. (C) Carefully place a cover glass over the small well. (D) Add agarose layer 2 on the whole bottom of the 35 mm dish. (E) Add E3 to the dish. (F) Schematic drawing of a cross section of the mounting set up. (G) Microscope image (5x objective) of the zebrafish embryo in the final montage.
Materials
| Low melting agarose | Sigma-Aldrich, MO | A9414 | Store dissolved solution at 4 °C |
| 35 mm glass bottom dishes with No. 0 coverslip and 10 mm diameter of glass bottom | MatTek Corporation, MA | P35GCOL-0-10-C | |
| Tricaine (MS-222) | Sigma-Aldrich, MO | E10521 | Store dissolved solution at 4 °C |
| N-phenylthiourea (PTU) | Sigma-Aldrich, MO | P7629 | Store dissolved solution at -20 °C |
| DMC4500 digital microscope camera | Leica | NA | |
| Micro cover glass 22×22 mm | VWR | 48366 067 |

