Glioblastoma Induction Using SB Mediated Transposition: A Technique for Generating Novel Mouse Model Using Transposon Mediated Integration of SB Plasmid into Neonatal Mouse Brain
Abstract
Source: Calinescu, A. A. et al. Transposon Mediated Integration of Plasmid DNA into the Subventricular Zone of Neonatal Mice to Generate Novel Models of Glioblastoma. J. Vis. Exp. (2015)
This video describes an efficient technique to induce glioblastomas (GBM) using transposon DNA injected into the ventricles of neonatal mice. Cells of the subventricular zone, which take up the plasmid, transform, proliferate, and generate tumors with histopathological characteristics of human GBM.
Protocol
1. Intra-ventricular Neonatal Injections
- Three to four weeks prior to experiment, set up a mouse breeding cage with one male and one female mouse, and a plastic colored igloo. This provides an enriched environment and aids in the mating process.
- When pregnancy is confirmed, remove the male to a new cage. Eighteen days after mating, monitor cage daily for delivery. Perform intraventricular injections on postnatal day 1(P1).
- Preparation of the injection solution.
NOTE: The injection solution is made by mixing the plasmid DNA with the transfection reagent to create the particles for transfection. Below is an example on the preparation of an injection solution using a plasmid encoding the Sleeping Beauty transposase and luciferase: pT2/SB100x-Luc and two transposon plasmids: pT/CAGGS-NRASV12 and pT/CMV-SV40-LgT, at a ratio of 1:2:2. All the plasmids have a concentration of 2 µg/µL.- Prepare the DNA solution by adding: 4 µg (2 µL) of pT2/SB100x-Luc, 8 µg (4 µL) of pT/CAGGS-NRASV12 and 8 µg (4 µL) of pT/CMV-SV40-LgT and 10 µL of 10% glucose to a final volume of 20 µL at a concentration of 1 µg /mL DNA and 5% glucose.
- Prepare the PEI solution by adding 2.8 µL of in vivo jet PEI, 7.2 µL of sterile water, and 10 µL of 10% glucose to a final concentration of 5% glucose.
- Add the PEI solution to the DNA solution, mix and vortex and let sit at room temperature (RT) for 20 min. The solution is now ready for injection. After one hour at RT, keep the solution on ice.
- Fit a 10 µL clean syringe equipped with a 30G hypodermic needle with 12.5° bevel into the micropump (Figure 1 #1).
- To verify proper functioning of the injection system, use the automatic injector (Figure 1 #1) to withdraw 10 µL of water into the syringe and then empty the syringe completely.
- Cool the neonatal stereotaxic stage (Figure 1 #3) with a slurry of dry ice and alcohol to a temperature of 2–8°C.
- Induce anesthesia by placing the pups on wet ice for 2 min. Anesthesia will continue on the chilled stereotaxic frame for the remainder of the procedure. Maintaining the temperature of the frame above freezing, between 2–8 °C will prevent thermal burn injuries. As special precaution pups can be wrapped in gauze before placing on wet ice.
- Fill the syringe with the DNA/PEI solution.
- Immobilize the pup in the stereotaxic frame by placing its head between the gauze covered ear bars (Figure 1b). Ensure that the dorsal side of the skull is horizontal, parallel to the surface of the frame and that the cranial sutures are clearly visible. Wipe the head with 70% ethanol.
- Lower the needle of the syringe and adjust stereotaxic coordinates using dials of the stereotaxic frame until the needle touches the lambda (the midline intersection of the parietal and occipital bones) (Figure 1b).
- Lift the needle and adjust stereotaxic coordinates to 0.8 mm lateral and 1.5 mm rostral to the lambda (Figure 1b).
- Lower the needle until it touches and slightly dimples the skin. Measure the coordinates. Lower the needle another 1.5 mm. The needle will pierce the skin and skull, and pass through the cortex to enter the lateral ventricle (Figure 1c).
- Using the automatic injector, inject 0.75 µL of the DNA/PEI solution at a rate of 0.5 µL/min.
- Keep the pup on the frame for another minute to allow for the solution to disperse into the ventricles. In the meantime, anesthetize another pup, on ice for 2 min in preparation for the next injection.
- Gently and slowly lift the needle, and place the pup under a heating lamp. Monitor breathing and activity. If necessary, provide gentle stimulation of the limbs until first breathing ensues.
- Return the pup to its mother after it has warmed up, has reached a rosy color, is breathing regularly and is active, normally 5–7 min. If more than one pup is injected at a time, keep all pups together until all the injections are done. If injections take longer than 30 min, return half of the pups after the first 30 min and the rest at the end of the procedure.
- Monitor that the dam is responsive and nurturing to her pups. If necessary, add a surrogate mom to the cage.
- Ensure that the interval between placing the pup on ice and placing it under the warming lamp is less than 10 min. The quicker the procedure the better the survival. Usually, the time it takes to anesthetize and inject a pup is 4 min.
Representative Results
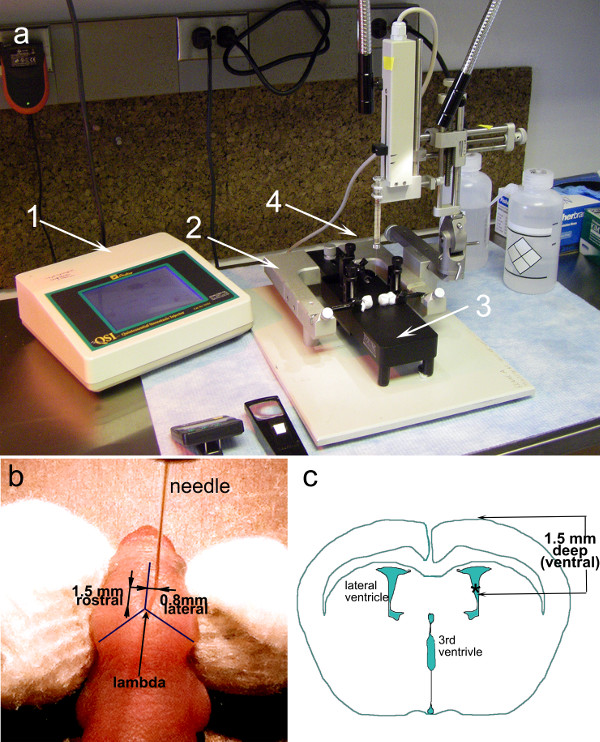
Figure 1: Experimental setup and guides for intra-ventricular injections in neonatal mice. (a) A stereotaxic frame (2) with micrometer dials is fitted with an automatic injector holding a 10 µl syringe (4). Inside the U frame, a neonatal adaptor frame is securely fastened (3). The control panel of the automatic injector (1) allows for precise selection of syringe, volume, and rate of flow. (b) Photograph of a neonatal mouse (P1) with a needle inserted at the coordinates required for injections into the lateral ventricle: 1.5 mm ventral and 0.8 mm lateral to the lambda. (c) Illustration of a coronal section through the brain of a neonatal mouse (P1) highlighting the relative dimensions and position of the ventricles.
Disclosures
The authors have nothing to disclose.
Materials
| Stoelting's Lab Standard with Rat and Mouse Adaptors | Stoelting | 51670 | Other companies produce similar frames, any of them with small mouse adaptors are suitable |
| Quintessential Stereotaxic Injector (QSI) | Stoelting | 53311 | Injections can be made without it, ,but the automatic injector allows for increased reproducibility and convenience |
| 10 μL 700 series hand fitted MICROLITER syringe | Hamilton | Model 701 | This syringe model will deliver from 0.5 to 500 μL of solution reliably. Other syringes (for example 5 μL , Model 75) may also be used |
| 30 gauge hypodermic needle (1.25", 15^{o} bevel) | Hamilton | S/O# 197462 | This needle is ideal to pierce the skin and the skull of a neonatal mouse without the need of other invasive procedures. If it gets dull (doesn't easily enter the skin), it needs to be replaced. |
| In vivo jet PEI | Polyplus Transfection | 201-10G | Aliquot in small volumes and keep at -20ºC |
| D-Luciferin, Potassium Salt | Goldbio.com | LuckK-1g | Other sources of firefly lucifierase are just as adequate |
| Hematoxylin Solution, Harris Modified | Sigma Aldrich | HHS128-4L |

