The Gliding Actin Filament Assay: An In Vitro Motility Assay to Study Translocation of Actin Filaments on Immobilized Myosins
Abstract
Source: Tripathi, A., et al. Myosin-Specific Adaptations of In vitro Fluorescence Microscopy-Based Motility Assays. J. Vis. Exp. (2021).
In this video, we perform an in vitro motility assay to analyze the actin-myosin interactions. The gliding movement of actin filaments on immobilized myosin molecules is visualized using fluorescence microscopy.
Protocol
1. Gliding actin filament assay
- Coverslip preparation
- Make a 1% nitrocellulose solution in amyl acetate.
- Obtain a tissue culture dish (150 x 25 mm) and add a circular filter paper (125 mm diameter) to the bottom of the dish.
- Load eight No. 1.5 thickness 22 mm square coverslips onto a rack and wash with approximately 2-5 mL of 200-proof ethanol followed by 2-5 mL of distilled water (dH2O). Repeat this washing step, ending with water. Then, dry the coverslips completely using a filtered air line or N2-line.
- Take one coverslip and slowly pipette 10 µL of the 1% nitrocellulose solution along one edge of the slip. Then, in one smooth motion, smear it across the rest of the coverslip using the side of a smooth-sided 200 µL pipette tip. Place this coverslip on the tissue culture dish with the nitrocellulose side up. Repeat for the remaining coverslips and allow them to dry while preparing the remaining reagents, and use coverslips within 24 h after coating.
- Chamber preparation
- Wipe a microscope slide with an optical lens paper to clean off large debris. Cut two pieces of double-sided tape, approximately 2 cm in length.
- Place one piece along the middle of the long edge of the microscope slide. Ensure that the edge of the tape aligns with the edge of the slide. Place the second piece of tape roughly 2 mm below the first piece of tape such that the two are parallel and aligned. This creates a flow chamber that can hold approximately 10 µL of solution (see Figure 1).
- Take one of the nitrocellulose-coated coverslips from Part 1. Carefully stick the coverslip onto the tape such that the side coated with nitrocellulose is making direct contact with the tape, (see Figure 1). Using a pipette tip, gently press down on the slide-tape interface to ensure that the coverslip has properly adhered to the slide. Cut the excess tape hanging over the edge of the slide with a razor blade.
- Actin preparation
- Make 20 µM F-actin by polymerizing globular actin (G-actin) in polymerization buffer (50 mM KCl, 2 mM MgCl2, 1 mM DTT, 25 mM MOPS (pH 7.0) at 4 °C overnight.
- Dilute F-actin to 5 µM in motility buffer (20 mM MOPS, 5 mM MgCl2, 0.1 mM EGTA, 1 mM DTT (pH 7.4)). Label with at least 1.2x molar excess of rhodamine-phalloidin. Leave (covered in aluminum foil) for at least 2 h on ice. This can be used for up to 1-2 months, and stored on ice.
- Performing the myosin 5a gliding actin filament assay
NOTE: In this section, the details of the myosin 5a (HMM) gliding assay are provided.- Prepare the solutions for myosin 5a described in Table 1 and keep them on ice.
- Flow in 10 µL of the myosin 5a (50-100 nM) through the flow chamber and wait for 1 min.
- Flow in 10 µL of the 1 mg/mL BSA in 50 mM MB with 1 mM DTT ("low salt" buffer). Repeat this wash two more times and wait for 1 min after the third wash. Use the corner of a tissue paper or filter paper to wick the solution through the channel by gently placing the corner of the paper at the flow chamber exit.
- Wash with 10 µL of 50 mM MB with 1 mM DTT. Repeat this wash two more times.
- Flow in 10 µL of the black actin solution (5 µM F-actin, 1 µM calmodulin, and 1 mM ATP in 50 mM MB with 1 mM DTT) to eliminate "dead heads".
- Pipette the solution with a 1 mL syringe and 27 G needle to shear the actin filaments before introducing the solution to the chamber. Repeat this step two more times and wait for 1 min after the third time. Approximately 20 pipetting events are sufficient.
- To perform the "dead head" spin, add a stoichiometric amount of F-actin to myosin in the presence of 1 mM ATP and 1 mM MgCl2 at a salt concentration of 500 mM. Then ultracentrifuge at 480,000 x g for 15 min at 4 °C. The dead myosin will be in the pellet.
- Flow in 50 µL of 50 mM MB with 1 mM DTT and 1 mM ATP to deplete the chamber of free actin filaments.
- Wash with 10 µL of 50 mM MB with 1 mM DTT. Repeat this wash two more times to deplete the chamber of any ATP.
- Flow in 10 µL of 20 nM rhodamine actin (Rh-Actin) solution containing 1 mM DTT in 50 mM MB and wait for 1 min to allow rigor binding of actin filaments to the myosin 5a attached to the surface of the coverslip.
- Wash with 10 µL of 50 mM MB with 1 mM DTT to wash away Rh-Actin filaments not bound to the surface. Repeat this wash two more times.
- Flow in 30 µL of Final Buffer.
- Record images on a fluorescence microscope using an excitation wavelength of 561 nm to visualize Rh-Actin. An appropriate exposure time is 200 ms at 1.4 mW laser power for a total acquisition duration of 0.5-1 min.
NOTE: Ensure that the acquisition rate is scaled appropriately to the speed of the moving filaments. An important consideration before collecting data for use with tracking programs is the acquisition frame rate. Subpixel movements between frames will result in an overestimate of the velocity, and movements of several hundred nanometers are required to obtain accurate values. An optimal acquisition rate features actin gliding for at least a one-pixel distance between frames. In the case of the TIRF microscope used for the imaging here, this threshold translates to 130 nm; therefore, a myosin expected to travel 1 µm/s must be imaged at a rate of 5 frames/s (0.2 s interval) to achieve 200 nm of movement while a myosin expected to travel 10 nm/s requires 0.05 frames/s (20 s intervals). Data can therefore be downsampled at this stage if necessary.
Table 1: Buffers used in gliding assay
| Buffer Name | Composition (M5a) | Composition (NM2b) | Step(s) Used (M5a/NM2b) | Comments |
| 4X Motility Buffer (4X MB) | 80 mM MOPS, pH 7.2 | 80 mM MOPS, pH 7.2 | Vacuum filter and store in 4°C | |
| 20 mM MgCl2 | 20 mM MgCl2 | |||
| 0.4 mM EGTA | 0.4 mM EGTA | |||
| pH 7.4 | pH 7.4 | |||
| 50 mM Salt Motility Buffer (50 mM MB) | 25% v/v 4X MB | 25% v/v 4X MB | Vacuum filter and store in 4°C | |
| 50 mM KCl | 50 mM NaCl | |||
| Raise to volume with dH2O | Raise to volume with dH2O | |||
| 500 mM Salt Motility Buffer (500 mM MB) | N/A | 25% v/v 4X MB | Vacuum filter and store in 4°C | |
| 500 mM NaCl | ||||
| Raise to volume with dH2O | ||||
| Myosin | 0.05-0.1 µM myosin | 0.2 µM myosin | 4.2 | Keep on ice. |
| 1 mM DTT | 1 mM DTT | |||
| Dilute in 50 mM MB | Dilute in 500 mM MB | |||
| 1 mg/mL Bovine Serum Albumin (BSA) | 1 mg/mL BSA | 1 mg/mL BSA | 4.3 | Keep on ice. |
| Dilute in 50 mM MB | Dilute in 500 mM MB | |||
| 1 mM DTT | 1 mM DTT | |||
| 5 µM Unlabeled F-actin in 50 mM MB (black actin) | 5 µM unlabeled F-actin | 5 µM unlabeled F-actin | 4.5 | Keep on ice. Shear actin by pipetting up and down 5-10 times, or by using a syringe. |
| 1 μM calmodulin (CaM) | 1 mM ATP | |||
| 1 mM ATP | 0.2 mM CaCl2 | |||
| Dilute in 50 mM MB | 1 μM CaM | |||
| 1–10 nM myosin light chain kinase (MLCK) | ||||
| Dilute in 50 mM MB | ||||
| MB with 1 mM DTT and 1 mM ATP | 1 mM DTT | 1 mM DTT | 4.6 | Keep on ice. |
| 1 mM ATP | 1 mM ATP | |||
| Dilute in 50 mM MB | Dilute in 50 mM MB | |||
| MB with DTT | 1 mM DTT | 1 mM DTT | 4.4, 4.7, 4.9 | Keep on ice. |
| Dilute in 50 mM MB | Dilute in 50 mM MB | |||
| 20 nM Rhodamine-Phalloidin F-actin (Rh-Actin) | 20 nM Rhodamine-phalloidin F-actin | 20 nM Rhodamine-phalloidin F-actin | 4.8 | Keep on ice. Do not vortex. |
| 1 mM DTT | 1 mM DTT | |||
| Dilute in 50 mM MB | Dilute in 50 mM MB | |||
| Final Buffer | 50 mM KCl | 0.7% methylcellulose (optional) | 4.10 | Add in the glucose, glucose oxidase, and catalase immediately before performing the experiment. Keep on ice. |
| 20 mM MOPS, pH 7.2 | 50 mM NaCl | |||
| 5 mM MgCl2 | 20 mM MOPS, pH 7.2 | |||
| 0.1 mM EGTA | 5 mM MgCl2 | |||
| 1 mM ATP | 0.1 mM EGTA | |||
| 50 mM DTT | 1 mM ATP | |||
| 1 μM calmodulin | 50 mM DTT | |||
| 2.5 mg/mL glucose | 1–10 nM MLCK | |||
| 100 μg/mL glucose oxidase | 0.2 mM CaCl2 | |||
| 40 μg/mL catalase | 1 μM calmodulin | |||
| 2.5 mg/mL glucose | ||||
| 100 μg/mL glucose oxidase | ||||
| 40 μg/mL catalase |
Representative Results
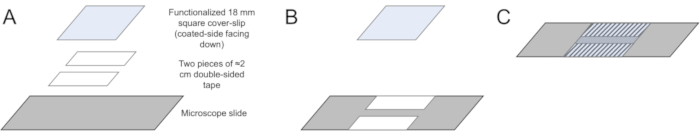
Figure 1: Preparation of functionalized flow-cell chambers. (A) Begin with a cleaned microscope slide, two pieces of double-sided tape cut to approximately 2 cm, and a functionalized coverslip. (B) Add the tape to the center of the microscope slide. (C) Attach the coverslip to the tape with the coating (i.e., nitrocellulose) facing down and gently press on the overlapping regions with the tape using a plastic pipette tip to ensure that the coverslip has adhered to the chamber.
Disclosures
The authors have nothing to disclose.
Materials
| Amyl Acetate | Ladd Research Industries | 10825 | |
| ATP | Millipore Sigma | A7699 | |
| Bovine Serum Albumin | Millipore Sigma | 5470 | |
| Calmodulin | PMID: 2985564 | ||
| Catalase | Millipore Sigma | C40 | |
| Circular Filter Paper – Gliding Assay | Millipore Sigma | WHA1001125 | |
| Coverslip Rack | Millipore Sigma | Z688568-1EA | |
| Coverslips: Gliding Acting Filament Assay | VWR International | 48366-227 | |
| DL-Dithiothreitol | Millipore Sigma | D0632 | |
| Double-Sided Tape | Office Depot | 909955 | |
| EGTA | Millipore Sigma | E4378 | |
| Ethanol | Fischer Scientific | A4094 | |
| G-actin | PMID: 4254541 | G-actin stock can be stored at 200 μM in liquid N2. | |
| Glucose | Millipore Sigma | G8270 | |
| Glucose Oxidase | Millipore Sigma | G2133 | |
| KCl | Fischer Scientific | P217-500 | |
| Large-Orifice Pipet Tips | Fischer Scientific | 02-707-134 | |
| Methylcellulose | Millipore Sigma | M0512 | |
| Microscope Slides | Fischer Scientific | 12-553-10 | |
| MOPS | Fischer Scientific | BP308-100 | |
| Nitrocellulose | Ladd Research Industries | 10800 | |
| Razor Blades | Office Depot | 397492 | |
| Rhodamine-Phalloidin | ThermoFisher Scientific | R415 | Stock can be diluted in 100% methanol to a final concentration of 200 μM. |
| Tissue Culture Dish – Gliding Assay | Corning | 353025 | Each tissue culture dish can hold approximately nine coverslips. |
| Smooth-sided 200 µL Pipette Tips | Thomas Scientific | 1158U38 | |
| EQUIPMENT | |||
| Centrifuge | ThermoFisher Scientific | 75006590 | |
| Microscope | Nikon | Model: Eclipse Ti with H-TIRF system with 100x TIRF Objective (N.A. 1.49) | |
| Microscope Camera | Andor | Model: iXon DU888 EMCCD camera (1024 x 1024 sensor format) | |
| Microscope Environmental Control Box | Tokai HIT | Custom Thermobox | |
| Microscope Laser Unit | Nikon | LU-n4 four laser unit with solid state lasers for 405nm, 488nm, 561nm, and 640nm | |
| Optima Max-Xp Tabletop Ultracentrifuge | Beckman Coulter | 393315 |

