Near-Infrared Photoimmunotherapy for Targeted Cell Elimination in Mixed 3D Cultures
Abstract
Source: Sato, K. et al., Selective Cell Elimination from Mixed 3D Culture Using a Near Infrared Photoimmunotherapy Technique. J. Vis. Exp. (2016)
This video demonstrates a technique for selectively removing specific cells from a mixed 3D cell culture through near-infrared photoimmunotherapy. In this method, targeted cells are accurately identified using an antibody conjugated with an NIR photoabsorber. Subsequent irradiation with infrared light induces cell death, effectively eliminating the targeted cells from the 3D culture.
Protocol
NOTE: The following protocol describes the necessary steps to eliminate specific cells using Near infrared photoimmunotherapy (NIR-PIT). Controls and other details about NIR-PIT and cell viability can be found elsewhere.
1. Conjugation of Infrared 700 (IR700) to Monoclonal Antibodies (mAb)
- Prepare mAb of interest at 2-5 mg/ml in 0.1 M disodium hydrogen phosphate (Na2HPO4) (pH 8.6) solution.
- Mix 6.8 nmol of mAb with 30.8 nmol of 10 mM IR700 in 0.1 M Na2HPO4 solution (pH 8.6) in a microcentrifuge tube and incubate at RT for 1 hr, covered with aluminum foil.
- Wash the PD-10 column (see Table of Materials/Equipment) with 15 ml PBS, twice. Load the sample from step 1.2.
- Purify the mixture via the PD-10 column by PBS elution according to manufacturer's instructions.
NOTE: Here, the elution was along the band color of IR700. For a 300 µl sample, PBS elution fraction is typically between 2.5 ml to 4.4 ml. - Determine the protein concentration with Coomassie staining by measuring the absorption at 595 nm with a spectrophotometer. Determine the concentration of IR700 with absorption at 689 nm to confirm the number of fluorophore molecules conjugated to each mAb molecule.
NOTE: It is important to determine an optimal conjugation number of IR700 molecules in 1 mAb with a spectrophotometer. Generally, around 3 IR700 molecules in 1 mAb molecule is optimal for both in vitro and in vivo work. High-performance liquid chromatography (HPLC) and sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) methods can be used to confirm whether the mAb and IR700 are bound or not. - Store at 4 °C after determining the protein concentration.
2. Preparation of Mixed 3D Cell Culture (Mixed Spheroid)
- Apply sterile water (around 1 ml) into the plate reservoir section of hanging drop plates.
- Prepare various ratios of the cell types of interest — A431-luciferase-green fluorescent protein (A431-luc-GFP) cells and 3T3-red fluorescent protein (3T3-RFP) cells — with each sample containing 5,000 cells total, suspended in 50 µl of culture media.
NOTE: Prepare 1:100, 10: 100, 25:100, 50:100, etc. ratios of cells types of interest in culture media depending on the cell-type. - Incubate the mix for 5-7 days in the 96 well hanging drop plate in a humidified incubator at 37 °C and 5% carbon dioxide. Change the culture media every 2 days. NOTE: To make the 3D spheroid precisely, handle the plate gently, as drops containing cells fall off easily before forming 3D shapes.
- Observe the morphology and size of the spheroids using an inverted brightfield microscope at 10X – 40X magnification. NOTE: Although it depends on cell types and the size of hanging-drop, ensure that the diameter of the spheroid is around 400-600 µm after 7 days of incubation in the 96 well hanging drop plate.
3. In Vitro NIR-PIT for Mixed 3D Cell Culture
- Change the media of the hanging drop plates to 10 µg/ml antibody-photoabsorber conjugate (APC) containing media, and incubate for 6 hr in a humidified incubator at 37 °C and 5% carbon dioxide.
- After 6 hr incubation, wash the spheroid twice with fresh culture media (phenol red free). Gently transfer the spheroid to a glass-bottomed 50 mm dish with 100 µl fresh phenol red free culture media using a sterile 200 µl pipette tip with the tip cut off. Place one spheroid in each dish.
- Observe the spheroid with an inverted brightfield microscope to detect the change of morphology. To observe the optical reporters (e.g., GFP and RFP) use fluorescence microscope with the following filter settings: GFP — 469 nm excitation filter, and 525 nm emission filter; RFP — 559 nm excitation filter, and 630 nm emission filter.
- Place the light-emitting diode (LED) above the glass-bottomed dish for irradiation.
NOTE: Spheroids can be exposed to NIR light either on the microscope or in the laminar hood.- Measure the power density of the NIR-light with an optical power meter. According to this measurement, irradiate NIR-light via light-emitting diodes (LEDs) which emit light at wavelengths of 670 to 710 nm at 2 J/cm2.
NOTE: LED light can penetrate maximum depth of approximately 5 inches. Cytotoxic effects of NIR-PIT is dependent only on given energy regardless of power density and duration of exposure.
- Measure the power density of the NIR-light with an optical power meter. According to this measurement, irradiate NIR-light via light-emitting diodes (LEDs) which emit light at wavelengths of 670 to 710 nm at 2 J/cm2.
- After irradiation, transfer the spheroid into a new hanging drop plate with 50 µl of fresh culture media and incubate for 1 day in a humidified incubator at 37 °C and 5% carbon dioxide.
- Gently transfer the spheroid to a glass-bottomed dish with 100 µl of fresh culture media (phenol red free) using a sterile 200 µl pipette tip with the tip cut off. Observe the spheroid with a fluorescence microscope 1 day after NIR-PIT using filter settings described in step 3.3.
- Detect dead cells by adding propidium iodide to the media at a final concentration of 2 µg/ml, or by loss of cytoplasmic GFP-fluorescence using a fluorescence microscope (Figure 1B).
- Repeat steps 3.1-3.6 if target cells are not completely eliminated.
Representative Results
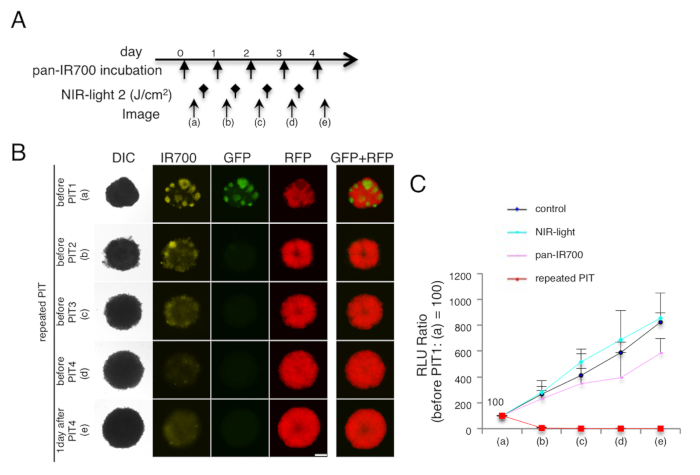
Figure 1. Target cell elimination in 3D cell spheroids (mixed spheroids of A431-luc-GFP and 3T3-RFP cells). (A) NIR-PIT (2 J/cm2) regimen is shown. (B) Repeated NIR-PIT completely eliminated target cells (A431-luc-GFP) with no harm to non-target cells (3T3-RFP), in a mixed 3D spheroid. Bar = 200 µm. (C) Quantification of luciferase activities (RLU ratio) demonstrated complete elimination of target cells (n = 10 spheroids in each group). Data are expressed as means ± s.e.m.
Disclosures
The authors have nothing to disclose.
Materials
| IRDye 700DX Ester Infrared Dye | LI-COR Bioscience (Lincoln, NE, USA) | 929-70011 | |
| Na2HPO4 | SIGMA-ALDRICH (St. Louis, MO, USA) | S9763 | |
| Sephadex G25 column (PD-10) | GE Healthcare (Piscataway, NJ, USA) | 17-0851-01 | |
| Coomassie (bradford) Plus protein assay | Thermo Fisher Scientific Inc (Waltham, MA, USA) | PI-23200 | |
| Perfecta3D 96-Well hanging Drop Plates | 3D Biomatrix Inc (Ann Arbor, MI, USA) | HDP1096-8 | |
| Optical power meter | Thorlabs (Newton, NJ, USA) | PM100 | |
| LED: L690-66-60 | Marubeni America Co. (Santa Clara, CA, USA) | L690-66-60 | |
| Vectibix (panitumumab) | Amgen (Thousand Oaks, CA, USA) | ||
| 35mm glass bottom dish, dish size 35mm, well size 10mm | Cellvis (Mountain View, CA, USA) | D35-10-0-N |

