Abstract
Source: Haque, M. et al., Development of Stem Cell-derived Antigen-specific Regulatory T Cells Against Autoimmunity. J. Vis. Exp. (2016)
This video showcases a cell-based therapy for autoimmune arthritis in mice. iPSC-derived T regulatory cells expressing ovalbumin-specific receptors are injected with methylated bovine serum albumin (mBSA) emulsified in an adjuvant. Murine antigen-presenting cells (APCs) process and present the mBSA to T cells, prompting T cell activation and migration to the knee joint's synovial membrane. Subsequent injections involve mBSA alone in the left knee and a combination of mBSA and ovalbumin in the right knee. In the left knee, the immune response to mBSA triggers arthritis, while in the right knee, T-regulatory cells interacting with ovalbumin mitigate inflammation, improving arthritis symptoms.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. In Vivo Maturation and Suppression of Autoimmune Arthritis
- Generate the constructs to be used in retroviral transduction by sub-cloning the OT-II T cell receptor (TCR) genes and forkhead box protein 3 (FoxP3) into the MiDR plasmid linked with self-cleaving peptide 2A to make the MiDR-TCRα-2A-TCRβ-2A-FoxP3 construct.
- Perform retroviral transduction of induced pluripotent stem cells (iPSCs) using Plat E cells as the packaging cell line.
- In vivo differentiation and arthritis induction in mice.
- Differentiate OT-II TCR/FoxP3-transduced iPSCs (OT-II-FoxP3/iPSCs), FoxP3-transduced iPSCs, and DsRed transduced iPSCs on the OP9-DL1-DL4-I-Ab stromal cells in the presence of cytokines mFlt-3L and mIL-7 for 8 days.
- On day 0, seed OP9-DL1-DL4-I-Ab cells at a minimum density of 104 cells/cm2 by using OP-9 media (α-MEM media containing 20% fetal calf serum (FCS) and 2.2 g/L sodium bicarbonate. Plate 1 x 106 cells per 10 cm dish.
- On day 3, when OP9-DL1-DL4-I-Ab cells become 80-90% confluent, remove the media and seed 0.5 – 1 x 105 iPSCs over OP9-DL1-DL4–I-Ab cells in OP-9 media.
NOTE: This establishes the co-culture of iPSCs on OP9-DL1-DL4-I-Ab cells and is considered day 0 of differentiation. - On day 5, remove the media from the 10 cm dish by aspiration, wash the cells with 10 ml of 1x phosphate-buffered saline (PBS), and aspirate the PBS. Add 4 ml of 0.25% trypsin and incubate at 37 °C for 10 min. Add another 8 ml of iPSC media to the cells, re-suspend them, and centrifuge at 400 x g for 5 min at room temperature.
- Aspirate the supernatant and re-suspend the cells in 10 ml of iPSC media. Incubate these re-suspended cells on a fresh 10 cm dish and return to the incubator for 30 min.
NOTE: Remove the OP9-DL1-DL4-I-Ab feeder cells and keep the differentiating iPSCs in the media. Maintain OP9-DL1-DL4-I-Ab cells continuously to achieve 80 – 90% confluency for further co-culture. - After 30 minutes, collect the floating cells, filter them through a 70 µm cell strainer, and count them with a hemocytometer.
- Aspirate the supernatant and re-suspend the cells in 10 ml of iPSC media. Incubate these re-suspended cells on a fresh 10 cm dish and return to the incubator for 30 min.
- Seed 5 x 105 of iPSCs to a fresh 80 – 90% confluent of OP9-DL1-DL4-I-Ab cells in OP9 media. Supplement the media with mFlt-3L at a final 5 ng/ml concentration.
- Trypsinize all three kinds of cells from the 10 cm plate and re-suspend cells from each 10 cm plate in 10 ml of fresh media. Add the cells to a fresh 10 cm plate and return to the incubator for 30 min. After 30 min, collect floating cells.
- Pass cells through a 70 µm cell strainer to remove cell clusters and count using a hemocytometer. Adjust the cells to a concentration of 1.5 x 107 cells/ml in cold PBS and filter again if needed. Keep cells on ice until adoptive cell transfer into mice.
- Inject 200 µl of the cell suspension (3 x 106 cells) into three different groups of 4 – 6 week-old female C57BL/6 mice through the tail vein.
- On day 10, after the cell transfer, inject mice with 100 µg of methylated bovine serum albumin (mBSA) emulsified in Freund's complete adjuvant at the base of the tail using a 1 ml syringe.
- On day 17, anesthetize mice using an isoflurane vaporizer. Utilize 4 – 5% isoflurane for induction and 1 – 2% for maintenance. Induce arthritis by intra-articular injection of 20 µg mBSA in 10 µl PBS in the left knee joint and of 20 µg mBSA and 100 µg whole ovalbumin (OVA) in 10 µl PBS in the right knee joint. Food and a gel pack can be placed on the bedding floor after arthritis has developed. Mice will be euthanized: Body Condition Score (BCS) of 2/5 or moribund, severe cachexia and/or persistent recumbency (a 24-hr period), and severity score of 4. In score 4, erythema and severe swelling encompass the ankle, foot, and digits, or ankyloses of the limb.
- Characterization of the OT-II TCR/FoxP3-transduced iPSCs.
- Use a fluorescent microscope (20X) to visualize unfixed live DsRed+ GFP+ cells.
- Examine gene integration and expression by both Western blot and flow cytometry.
- Arthritis suppression assay
- Measure the swelling of both knees of the mice before arthritis induction by using a dial-gauge caliper to establish a baseline.
- Follow steps (1.3.1 – 1.3.10) for arthritis induction and Treg transfer. Measure mouse knees with a dial-gauge caliper post-Treg transfer.
- Calculate the percent increase in the knee diameter: Percent Increase = (Knee diameter on day 1 – Knee diameter on day 0) / Knee diameter on day 0.
- Measurement of joint destruction and inflammatory cell infiltration in the knees by histology (Figure 1).
- On day 7 post-arthritis induction, euthanize the mice by carbon dioxide (CO2) inhalation followed by cervical dislocation. Remove hair from the knees with an electric clipper and excise the knees by surgical procedure.
- Remove the skin from the legs by making an incision in the hind leg with blunt-end scissors and cut the skin across the thigh and down to the ankle. Gently peel the skin over the leg and foot to expose the muscle. Remove the muscle from the leg carefully without damaging the knee joint.
- Cut the hind leg just above the pelvic/hip joint and cut the tibia using sharp dissecting scissors.
- Fix the knees in 4% formalin for 48 hr. Decalcify the knees by using 2.5 M formic acid; rinse thrice in xylene for 3 min, rinse twice in 100% ethanol, rinse twice in 95% ethanol, rinse twice in deionized water for 2 min, and decalcify in 1 mM ethylenediaminetetraacetic acid (EDTA). Treat at a sub-boiling temperature (90 °C) for 20 min.
- Remove both knees, fix in 10% formalin, and calcify in Formical-4. Embed the tissues in Paraffin, section at 4 µm, and stain with hematoxylin and eosin (H & E) or Safranin O staining and observe under a microscope.
Representative Results
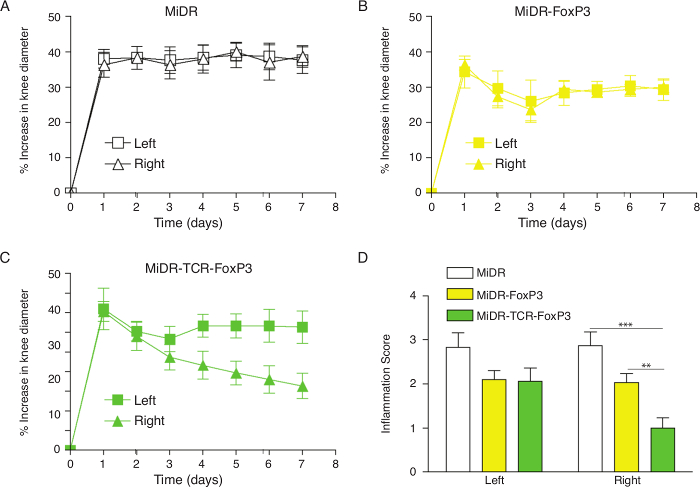
Figure 1: Adoptive Transfer of Ag-specific iPSC-Tregs Ameliorates AIA in Mice. Murine iPSCs were transduced with the retroviral construct MiDR, MiDR-FoxP3, or MiDR-TCR-FoxP3 and were co-cultured on the OP9-DL1/DL4/I-Ab cells. On day 7, the gene-transduced cells (3 × 106/mouse) were adoptively transferred into female C57BL/6 mice induced with AIA two weeks after the cell transfer. On the day following arthritis induction, the arthritis severity was monitored by measurement of the knee diameter. (A-C) Percent increase in knee diameter. An increase in knee diameter was calculated based on preinjection knee diameter for each mouse before injection on day 0. Arthritis score was evaluated by examining both knees in a blinded manner; each knee was assigned a score (0: no visible swelling or discoloration; 1: visible swelling with or without discoloration; 2: moderate swelling with discoloration; 3: severe swelling with discoloration). In each group, five mice were used, and the data are representative of three independent experiments. Data are represented as the mean ± SD. (D) The mean scoring on day 7 for both knees was from five mice. Data are represented as the mean ± SD from three independent experiments (** p< 0.01, *** p< 0.001, two-way ANOVA).
Disclosures
The authors have nothing to disclose.
Materials
| C57BL/6j mice | Jackson Laboratory | 664 | |
| α-MEM | Invitrogen | A10490-01 | |
| FBS | Hyclone | SH3007.01 | |
| Polybrene | Sigma | 107689 | |
| Genejammer | Integrated science | 204130 | |
| mFlt-3L | peprotech | 250-31L | |
| mIL-7 | peprotech | 217-17 | |
| mBSA | Sigma | A7906 | |
| Ova albumin | Avantor | 0440-01 | |
| CFA | Difco | 2017014 | |
| Tailveiner restrainer | Braintree scientific | RTV 150-STD | |
| Paraformaldehyde | Sigma | P6148-500G | Caution: Allergenic, Carcenogenic, Toxic |
Tags

.
