Electrospinning Fundamentals: Optimizing Solution and Apparatus Parameters
Summary
Electrospinning techniques can create a variety of nanofibrous scaffolds for tissue engineering or other applications. We describe here a procedure to optimize the parameters of the electrospinning solution and apparatus to obtain fibers with the desired morphology and alignment. Common problems and troubleshooting techniques are also presented.
Abstract
Electrospun nanofiber scaffolds have been shown to accelerate the maturation, improve the growth, and direct the migration of cells in vitro. Electrospinning is a process in which a charged polymer jet is collected on a grounded collector; a rapidly rotating collector results in aligned nanofibers while stationary collectors result in randomly oriented fiber mats. The polymer jet is formed when an applied electrostatic charge overcomes the surface tension of the solution. There is a minimum concentration for a given polymer, termed the critical entanglement concentration, below which a stable jet cannot be achieved and no nanofibers will form – although nanoparticles may be achieved (electrospray). A stable jet has two domains, a streaming segment and a whipping segment. While the whipping jet is usually invisible to the naked eye, the streaming segment is often visible under appropriate lighting conditions. Observing the length, thickness, consistency and movement of the stream is useful to predict the alignment and morphology of the nanofibers being formed. A short, non-uniform, inconsistent, and/or oscillating stream is indicative of a variety of problems, including poor fiber alignment, beading, splattering, and curlicue or wavy patterns. The stream can be optimized by adjusting the composition of the solution and the configuration of the electrospinning apparatus, thus optimizing the alignment and morphology of the fibers being produced. In this protocol, we present a procedure for setting up a basic electrospinning apparatus, empirically approximating the critical entanglement concentration of a polymer solution and optimizing the electrospinning process. In addition, we discuss some common problems and troubleshooting techniques.
Protocol
1. Choose a Polymer
- Choose a polymer (e.g., poly-L-lactic acid (PLLA), polycaprolactone (PCL), polystyrene (PS) or nylon) based on your specifications (e.g., biodegradable, thermoplastic or cross-linkable) and a solvent of that polymer. Choose suitable personal protective equipment based on your selection.
- Select a substrate based on your application (e.g., glass, plastic, metal or silicon wafer).
2. Choose a Collector
- Choose the collector geometry based on your specifications. Random fibers can be collected on stationary plates. Aligned fibers can be collected on rapidly rotating wheels, drums or rods, or on parallel plates.
- The collector must be conductive and must remain isolated from its axel in such a way that it can be grounded without also grounding adjacent objects, the table top, etc.
3. Approximate the Critical Entanglement Concentration Empirically1
- Prepare several candidate polymer concentrations (e.g., 4, 10, 15, 20, 30 wt%) and choose a concentration that flows (the solution should be a viscous liquid but not a gel) to start with.
- Set up the electrospinning apparatus2,3,4,5 (see Figure 1)
- Load the syringe pump and set the pump speed such that any bead of solution wiped from the tip is immediately replaced.
- Ground the collector and clip the high voltage wire to the conductor plate (a small square of conductive material such as aluminum foil through which the syringe tip protrudes).
- Start the wheel spinning.
- Make sure the power supply is set to zero before turning it on.
- Observe the stream
- Ramp voltage up slowly and watch the bead of the solution at the needle tip.
- Adjust the voltage to obtain a long and steady stream. If a steady stream cannot be obtained, adjust the polymer solution concentration. See Table 1 for an example.
4. Troubleshooting – the Stream:
- Trouble viewing the stream
- Use a dark matte backdrop and place a unidirectional light source (such as a flashlight) between the viewer and the stream (see Figure 2).
- Dripping from the syringe tip
- If the polymer solution is dripping straight down with no attraction to the wheel, make sure the conductor plate is making contact with the needle tip and that the collector is making contact with ground.
- If the drop of polymer solution at the syringe tip is leaning in the direction of the wheel but is not forming a stream, increase the voltage. The quality of the stream can be adjusted by varying the distance and the voltage until a steady stream is visible. See Figure 3 for suggested distances with corresponding voltages for a 4% PLLA solution and an 8×8 cm conductor plate.
- Large globs at the syringe tip
- When the polymer solution begins to collect and harden at the tip of the needle, swipe the glob away with a paper towel attached to a non-conductive stick.
- Oscillating or ‘wagging’ streams
When the stream is wagging up and down rapidly, turn down the voltage or increase the distance between the syringe tip and the wheel. If the stream continues to wag use a higher concentration of polymer or add a little solvent with a slower evaporation. - Short or discontinuous streams
Visible steady streams making observable contact with the wheel set at a high rotational velocity yield the highest quality of uniformity and alignment. When the stream is short and discontinuous, increasing the polymer solution, adding more slow evaporating solvent, and adjusting the voltage will improve the length and steadiness of the stream.
5. Troubleshooting – Fiber Morphology6,7,8 (refer to Figure 4)
- Beading
When beads are discovered in the fibers, increase the polymer solution and make sure the conductor plate is making continuous contact with the needle and that the grounded wire brush is making continuous contact with the wheel. - Ribbons and bleeding fibers
When fibers are forming as ribbons or are bleeding together, use a higher concentration of polymer or a solvent with a higher rate of evaporation (more volatile). - Curlicue or wavy fibers
When fibers are forming waves or curlicues, increase the wheel speed or move the needle tip further from the collector. Also, check that the conductor plate and collector are not vibrating. - Porosity9
If pores are desired, use a rapidly evaporating solvent. If pores are not desired, try adding a small amount of co-solvent that is less volatile than the major solvent. - Alignment10
When the collector is moving at a low RPM or at rest, the alignment quality is poor. Increase the alignment by increasing the speed of the wheel.
6. Representative Results:
Please refer to Figure 4 for depictions of typical fiber results.
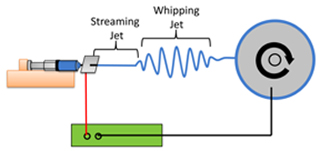
Figure 1. A typical electrospinning setup. A polymer solution (blue) is dispensed from a syringe pump (orange). A high voltage DC power supply (green) grounds a rapidly rotating wheel collector (grey) onto which aligned nanofibers are collected. The polymer jet between the syringe and collector consists of a steady streaming segment and a rapidly oscillating whipping segment.

Figure 2. The streaming jet is visible exiting the syringe tip; the whipping jet is too small to be seen.
Approximating the critical entanglement concentration of PLLA
| PLLA (% wt/v) | Observation | Concentration Adjustment |
| 0.5 | Dripping; no stream | Increase |
| 2.0 | Spitting small globs; no stream | Increse slightly |
| 4.0 | Steady stream | Good |
| 6.0 | Spitting large globs or beads | Decrease slightly |
| 12.0 | Clumping at the tip; no stream | Decrease |
Table 1. An example depicting the approximation of the critical entanglement concentration of PLLA. Various polymer concentrations are tried and the resulting streaming jets observed until a steady stream is obtained.
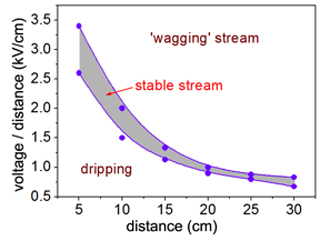
Figure 3. The distance between the syringe tip and the collector must be balanced with the applied voltage to obtain a steady streaming jet. Excess applied voltage causes an oscillating or ‘wagging’ jet to form that results in less well-aligned fibers. When the voltage is too low, no jet will form and the solution will only drip from the syringe tip. The purple shaded region above represents the voltage range over which a steady streaming jet can be obtained for PLLA as a function of syringe-to-collector distance.
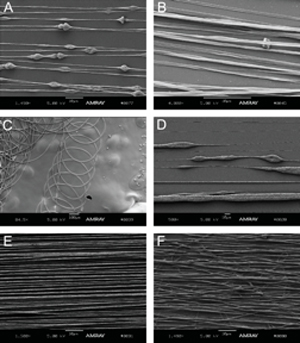
Figure 4. Electrospun fibers can exhibit a variety of morphologies, including beading (A), ribbons (B), curlicues (C), porous globs (D), good alignment (E) and poor alignment (F).
Discussion
Note: The majority of the examples presented here deal with electrospinning poly-L-lactic acid (PLLA) nanofibers. This is simply because PLLA is the most commonly spun polymer in our laboratory. However, we have also successfully used these methods to electrospin other polymers (e.g., PLGA, PCL, PS) and believe that the techniques presented here are easily applicable to the majority of mid- to high-molecular weight polymer solutions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH K08 EB003996 and the Paralyzed Veterans of America Research Foundation Grant 2573.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| High voltage DC power supply | Gamma High Voltage | ES40P-5W | ||
| Syringe pump | KD Scientific | KDS100 | ||
| Aluminum foil | Reynolds | |||
| Blunt metal tips, 23ga | Fisher | 13-850-102 | ||
| Polypropylene syringe | BD | 309585 | ||
| Rotating or stationary collector | Custom built | |||
| Various alligator clips and wires | ||||
| Dimethylformamide | Fisher | AC11622-0010 | ||
| Chloroform | Fisher | AC42355-0040 | ||
| PLLA | Boehringer Ingelheim | Resomer L210 | ||
| PLGA 85:15 | Sigma | 43471 | ||
| Carbon tape | Ted Pella | 13073-1 |
References
- Shenoy, S. L., Bates, W. D., Frisch, H. L., Wnek, G. E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: good solvent, non-specific polymer-polymer interaction limit. Polymer. 46, 3372-3384 (2005).
- Gertz, C. C., Leach, M. K., Birrel, L. K., Martin, D. C., Feldman, E. L., Corey, J. M. Accelerated neuritogenesis and maturation of primary spinal motor neurons in response to nanofibers. Dev. Neurobiol. 70, 589-603 (2010).
- Lin, D. Y., Johnson, M. A., Vohden, R. A., Chen, D., Martin, D. C. Tailored nanofiber morphologies using modulated electrospinning for biomedical applications. Mat. Res. Soc. Symp. Proc. 736, D3.8.1-D3.8.6 (2003).
- Corey, J. M., Gertz, C. C., Wang, B. S., Birrell, L. K., Johnson, S. L., Martin, D. C., Feldman, E. L. The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor and sensory neurons. Acta. Biomater. 4, 863-875 (2008).
- Corey, J. M., Lin, D. Y., Mycek, K. B., Chen, Q., Samuel, S., Feldman, E. L., Martin, D. C. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J. Biomed. Mater. Res. A. 83, 636-645 (2007).
- Tan, S. -. H., Kotaki, M., Ramakrishna, S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer. 46, 6128-6134 (2005).
- Yang, F., Murugan, R., Wang, S., Ramakrishna, S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 26, 2603-2610 (2005).
- Li, W., Laurencin, C. T., Caterson, E. J., Tuan, R. S., Ko, F. K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. A. 60, 613-621 (2002).
- Kim, C. H., Jung, Y. H., Kim, H. Y., Lee, D. R. Effect of collector temperature on the porous structure of electrospun fibers. Macromol. Res. 14, 59-65 (2006).
- Wang, H. B., Mullins, M. E., Cregg, J. M., Hurtado, A., Oudega, M., Trombley, M. T., Gilbert, R. J. Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J. Neural Eng. 6, (2009).

