Quantification of Proteins Using Peptide Immunoaffinity Enrichment Coupled with Mass Spectrometry
Summary
Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA) couples affinity enrichment of peptides with stable isotope dilution mass spectrometry (MRM-MS) to provide quantitative measurement of peptides as surrogates for their respective proteins. Here we describe the protocol using magnetic particles in a partially automated format.
Abstract
There is a great need for quantitative assays in measuring proteins. Traditional sandwich immunoassays, largely considered the gold standard in quantitation, are associated with a high cost, long lead time, and are fraught with drawbacks (e.g. heterophilic antibodies, autoantibody interference, ‘hook-effect’).1 An alternative technique is affinity enrichment of peptides coupled with quantitative mass spectrometry, commonly referred to as SISCAPA (Stable Isotope Standards and Capture by Anti-Peptide Antibodies).2 In this technique, affinity enrichment of peptides with stable isotope dilution and detection by selected/multiple reaction monitoring mass spectrometry (SRM/MRM-MS) provides quantitative measurement of peptides as surrogates for their respective proteins. SRM/MRM-MS is well established for accurate quantitation of small molecules 3, 4 and more recently has been adapted to measure the concentrations of proteins in plasma and cell lysates.5-7 To achieve quantitation of proteins, these larger molecules are digested to component peptides using an enzyme such as trypsin. One or more selected peptides whose sequence is unique to the target protein in that species (i.e. “proteotypic” peptides) are then enriched from the sample using anti-peptide antibodies and measured as quantitative stoichiometric surrogates for protein concentration in the sample. Hence, coupled to stable isotope dilution (SID) methods (i.e. a spiked-in stable isotope labeled peptide standard), SRM/MRM can be used to measure concentrations of proteotypic peptides as surrogates for quantification of proteins in complex biological matrices. The assays have several advantages compared to traditional immunoassays. The reagents are relatively less expensive to generate, the specificity for the analyte is excellent, the assays can be highly multiplexed, enrichment can be performed from neat plasma (no depletion required), and the technique is amenable to a wide array of proteins or modifications of interest.8-13 In this video we demonstrate the basic protocol as adapted to a magnetic bead platform.
Protocol
Experimental Procedure :
The assay requires synthetic peptides and anti-peptide antibodies. Selected peptides should be unique to the protein of interest, contain between 8 and 22 amino acids, and have no known post-translational modifications. Methionine residues are generally avoided and peptides containing dibasic amino acids (e.g. KK, KR, RR) are undesirable. For this technique, it is common to use stable isotope labeled peptides as internal standards, incorporating heavy (13C and 15N) labeled amino acids at the C-terminus of the peptide (i.e. K or R labeled).
The following protocol describes an assay developed to measure the peptide GDSLAYGLR from the mouse protein Osteopontin, using anti-peptide antibodies obtained from Epitomics Inc. (Burlingame, CA) and synthetic peptides from New England Peptide (Gardner, MA). The protocol consists of three main steps (Figure 1): 1) Trypsin digestion of the complex protein mixture, 2) Enrichment of peptides 3) Analysis by mass spectrometry. It will be demonstrated on a human plasma sample spiked with the mouse Osteopontin protein.
1. Trypsin enzymatic digestion and cleanup
- Thaw 10 μL neat plasma aliquot on wet ice.
- Determine the total protein concentration by BCA assay and centrifuge the sample to remove any suspended solids.
- Pipet 10 μL aliquot from its storage tube to a 1000 μL deepwell plate and cover with pierce-able film.
- Add 20 μL of fresh 9M urea / 30mM dithiothreitol (DTT) (final concentration 6M urea / 20mM DTT) to each sample.
- Incubate for 30 minutes at 37°C.
- Add 3 μL of fresh 500 mM iodoacetamide (final IAM 50mM) to each sample.
- Incubate for 30 minutes in the dark at room temperature.
- Add 257 μL of 100 mM Tris (pH 8) (dilutes urea to ˜0.6M).
- Add 10 μL of trypsin stock solution (1 μg/μL; for 1:50 enzyme:substrate ratio).
- Incubate 37°C overnight (12-16 hours).
- Add 3 μL of neat formic acid (final concentration of 1%).
- Add stable isotope standard (multiple standards are added if performing a multiplexed assay, typically this is about 10 μL containing 50-100 fmol of standard isotopically-labeled peptide).
- Wash the Oasis cartridge plate well with 500 μL of 0.1% formic acid in 80% acetonitrile, discarding the flow-through. Repeat this 3 times.
- Equilibrate the cartridge plate by adding 500 μL of 0.1% formic acid in water, and discard the flow-through. Repeat this 4 times.
- Load digest samples to the cartridge plate and adjust the vacuum so the flow is very slow.
- Wash with 500 μL of 0.1% formic acid in water, and discard flow-through. Repeat this 3 times.
- Elute peptides by adding 2 x 500 μL of 0.1% formic acid in 80% acetonitrile into 1000 μl deep-well plate (do not discard the flow-through).
- Lyophilize (or speedvac) the eluate to dryness. (Lyophilization is the preferred method)
- Reconstitute dried peptides by adding 50 μL PBS + 0.03% CHAPS.
2. Peptide immunoaffinity enrichment
- Transfer the sample to standard Kingfisher 96 well plates.
- Add 1 μg antibody and 1.5 μL Protein-G coated magnetic beads per target (it is optional to crosslink the antibody to the beads prior to addition). Ensure beads are well suspended by shaking or vortexing.
- Cover the plate with foil.
- Incubate overnight (12-16 hours) with gentle tumbling to ensure beads are suspended.
- Centrifuge plate at 32 x g for 5 seconds to remove any liquid from the foil surface.
- Remove the foil cover.
- Washing and elution of the beads can be performed manually or in an automated fashion. This procedure describes the steps automated on a Kingfisher platform.
- Wash the beads 2 times with 200 μL PBS + 0.03% CHAPS (1 minute per wash).
- Wash the beads 1 time with 200 μL 1:10 dilution of PBS + 0.03% CHAPS (1 minute).
- The peptides are eluted in 25 uL of 5% acetic acid + 0.03% CHAPS.
- Place the elution plate on a magnet and transfer the eluate to a 96 well plate, taking care not to transfer the leftover beads.
- Cover the plate with a sealing mat.
- The plate containing the eluate is delivered to the triple quadrapole mass spectrometer for analysis.
3. Analysis by multiple reaction monitoring – mass spectrometry
- Transitions for SRM/MRM analysis can be selected and optimized by infusing a 1 picomole per microliter solution of peptide standard in 30% acetonitrile/0.1% formic acid into the mass spectrometer at 0.5 μL/min. Once stable spray is obtained, collect an MS/MS spectrum.
- Transitions are selected from the MS/MS spectrum by identifying three abundant fragment ions in a region of the spectrum with little noise. For multiply-charged peptide ions, the y-ion fragments detected at m/z > precursor m/z are typically the best for SRM/MRM assay development. Figure 2 shows the MS/MS spectrum and selected transition ions 476.3 > 508.3, 579.3, 692.4 (transitions for the heavy stable isotope standard 481.3 > 518.3, 589.3, 702.4 not shown) for the peptide GDSLAYGLR.
- Depending on the make and model of the mass spectrometer used, there are multiple parameters that can be optimized for each transition. Here, we optimized the collision energy of each selected transition by ramping the collision energy and monitoring the level of signal. A collision energy of 25 was used for each transition.
- Briefly, a typical configuration for SRM/MRM analysis is as follows: Mobile phase (A) 0.1% Formic acid; (B) 90% Acetonitrile / 0.1% Formic acid, 0.3 x 5 mm C18 trap column, 75 μm ID x 15 cm C18 analytical column (Reprosil-Pur C18 AQ, 120 A° pore). The injection volume is 10 μL and the sample is loaded for 5 min at 3% B at a total flow rate 3 μL/min and eluted by a linear gradient from 3 – 45 %B at 300 – 400 nL/min in 10 min. Conditions on a 4000 QTRAP (ABSCIEX, Foster City, CA) were spray voltage 2.3kV, ion source temperature 150 °C, GS1 of 12, and curtain gas 15.
- The sample is analyzed by SRM/MRM by injecting 10 μL of the sample. Peak areas are integrated for light and heavy peptides using the program Skyline.14 Calculate the peak area ratio (PAR) of unlabeled/labeled peptide in each sample using the most abundant transition that is free of background noise, in this case, the y5 transition (light peptide 476.3 > 579.3, heavy standard peptide 481.3 > 589.3).
4. Representative Results:
Measured peak area ratios (light endogenous peptide relative to spiked heavy isotopically-labeled peptide) provide a quantitative measure of the target peptide. Figure 3 shows an example chromatogram of light and heavy peptides in a SISCAPA-enriched sample. Note that light and heavy peptides elute at the same time and multiple transitions can be monitored for each peptide to confirm the identity.
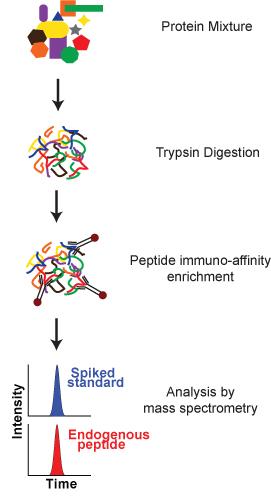
Figure 1. A schematic overview of the SISCAPA process. A complex protein mixture is digested into peptides. Targeted peptide analytes (endogenous analyte and a spiked stable isotope-labeled internal standard) are enriched using anti-peptide antibodies immobilized on Protein G-coated magnetic particles. Following isolation, the targeted peptides are eluted from the magnetic particles and analyzed by mass spectrometry for quantitation relative to the internal standard.
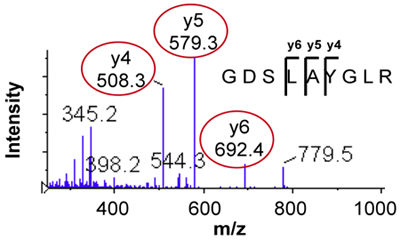
Figure 2. MS/MS spectrum of the peptide GDSLAYGLR showing selection of three fragments for SRM/MRM transitions.
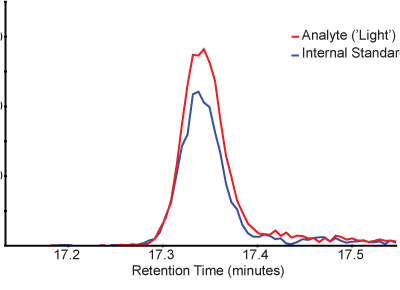
Figure 3. Example chromatograms showing the peak profile of a light peptide analyte (red) and the heavy stable isotope-labeled internal standard (blue). Chromatograms for the y5 transition from the light peptide (476.3 > 579.3) and heavy stable isotope labeled standard (481.3 > 589.3) are plotted over time as they elute from the chromatography system.
Discussion
The most critical step in the protocol as described is ensuring the beads remain well-mixed during the incubation period. Allowing the beads to settle to the bottom of the well/vial will result in increased variability. It is also important to spin-down any liquid that may remain on the top of the well/vial following the incubation period. Reproducible trypsin digestion is also critical. The procedure for digestion described here has been utilized extensively in our laboratory in conjunction with SISCAPA; however, it is likely alternate methods of digestion could be optimized for a given set of target proteins.15 Elution of free antibody does not interfere with detection of the peptide, likely because the antibody is excluded from the column or elutes later than the peptides, but the antibody can be crosslinked to the Protein G beads prior to affinity enrichment in large experiments or if free antibody becomes a problem. We have also found it useful to place a magnet below the sample plate on the autosampler as well as washing the trap column in the reverse direction of flow (compared to loading) to remove any residual beads or particles. In addition to the magnetic bead approach described, the technique can also be adapted to a column format (i.e. affinity chromatography).12, 16
Once the user is comfortable with the overall protocol, the technique is also amenable to several modifications that enhance the overall assay.11 First, it is possible to analyze multiple analytes in a single assay by combining antibodies in the enrichment step (i.e. multiplexing). The mass spectrometer is capable of simultaneously analyzing large numbers of analytes. Second, increasing the volume of original sample improves the sensitivity of the assay.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by the NCI Clinical Proteomic Technology Assessment Center (CPTAC) grant (#U24 CA126476) as well as a grant from the Entertainment Industry Foundation (EIF) and the EIF Women’s Cancer Research Fund to the Breast Cancer Biomarker Discovery Consortium, and generous gifts from the Keck Foundation, the Canary Foundation, and the Paul G. Allen Family Foundation.
Materials
| Name of the material | Company | Catalogue number |
|---|---|---|
| 1000ul Deep well 96-well plates | Eppendorf | 951032786 |
| Oasis HLB 1 cc cartridge plate, Sep-pak, 40 mg 96 well plate | Waters | 186003966 |
| Kingfisher 96 well plate | Thermo Fisher Scientific | 97002540 |
| Clear 96 well, white wall plate | Bio-Rad | HSP9601 |
| Foil cover for 96 well plate | Excelscientific | 12-169 |
| Axymat Sealing mat for 96 well plate | Axygen | 521-01-151 |
| X pierce film | Sigma-Aldrich | Z722502 |
| Kingfisher magnetic bead processor with PCR magnet head | Thermo Fisher Scientific | HSP 9601 |
Table 1: Materials
| Name of the material | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| Urea | Sigma Aldrich | U6031 | |
| Trizma base | Sigma Aldrich | T1503 | |
| Dithiothreitol (DTT) | Pierce | 20291 | “no-weigh dithiothreitol” |
| Iodoacetamide (IAM) | Sigma Aldrich | I1149 | |
| Trypsin Gold | Promega | V5280 | |
| Protein G magnetic beads, 2.8um | Invitrogen | 10004D | |
| Phosphate-buffered saline (PBS) | Thermo Fisher Scientific | L5401 | |
| CHAPS | Thermo Fisher Scientific | 28300 | |
| Formic acid | EMD | 11670 | |
| Acetonitrile | Thermo Fisher Scientific | A998-1 | |
| Acetic Acid | Sigma Aldrich | 242853 |
Table 2: Reagents
| Solution | Comments (optional) |
|---|---|
| 100 mM Tris pH 8 | |
| 9 M urea / 30 mM DTT in 100 mM Tris (pH 8) | must be prepared fresh each time |
| 500 mM IAM in 100 mM Tris (pH 8) | must be prepared fresh each time |
| Trypsin 1mg/mL in 100 mM Tris pH 8 | |
| Trypsin inhibitor 1mg/mL in 100 mM Tris pH 8 | |
| Stable isotope standard mastermix | |
| Anti-peptide antibody mastermix |
Table 3: Solutions to be prepared
References
- Hoofnagle, A. N., Wener, M. H. The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J. Immunol. Methods. 347, 3-11 (2009).
- Anderson, N. L. Mass spectrometric quantitation of peptides and proteins using Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA). J Proteome Res. 3, 235-244 (2004).
- Chace, D. H., Kalas, T. A. A biochemical perspective on the use of tandem mass spectrometry for newborn screening and clinical testing. Clin Biochem. 38, 296-309 (2005).
- Want, E. J., Cravatt, B. F., Siuzdak, G. The expanding role of mass spectrometry in metabolite profiling and characterization. Chembiochem. 6, 1941-1951 (2005).
- Barr, J. R. Isotope dilution–mass spectrometric quantification of specific proteins: model application with apolipoprotein A-I. Clin Chem. 42, 1676-1682 (1996).
- Gerber, S. A., Rush, J., Stemman, O., Kirschner, M. W., Gygi, S. P. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci U S A. 100, 6940-6945 (2003).
- Kuhn, E. Quantification of C-reactive protein in the serum of patients with rheumatoid arthritis using multiple reaction monitoring mass spectrometry and 13C-labeled peptide standards. Proteomics. 4, 1175-1186 (2004).
- Whiteaker, J. R. Antibody-based enrichment of peptides on magnetic beads for mass-spectrometry-based quantification of serum biomarkers. Anal Biochem. 362, 44-54 (2007).
- Hoofnagle, A. N., Becker, J. O., Wener, M. H., Heinecke, J. W. Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin Chem. 54, 1796-1804 (2008).
- Kuhn, E. Developing Multiplexed Assays for Troponin I and Interleukin-33 in Plasma by Peptide Immunoaffinity Enrichment and Targeted Mass Spectrometry. Clin Chem. 55, 1108-1117 (2009).
- Whiteaker, J. R., Zhao, L., Anderson, L., Paulovich, A. G. An automated and multiplexed method for high throughput peptide immunoaffinity enrichment and multiple reaction monitoring mass spectrometry-based quantification of protein biomarkers. Mol. Cell. Proteomics. 9, 184-196 (2010).
- Neubert, H., Gale, J., Muirhead, D. Online high-flow peptide immunoaffinity enrichment and nanoflow LC-MS/MS: assay development for total salivary pepsin/pepsinogen. Clin. Chem. 56, 1413-1423 (2010).
- Ackermann, B. L., Berna, M. J. Coupling immunoaffinity techniques with MS for quantitative analysis of low-abundance protein biomarkers. Expert Rev. Proteomics. 4, 175-186 (2007).
- MacLean, B. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 26, 966-968 (2010).
- Proc, J. L. A quantitative study of the effects of chaotropic agents, surfactants, and solvents on the digestion efficiency of human plasma proteins by trypsin. J. Proteome Res. 9, 5422-5437 (2010).
- Berna, M. Online immunoaffinity liquid chromatography/tandem mass spectrometry determination of a type II collagen peptide biomarker in rat urine: Investigation of the impact of collision-induced dissociation fluctuation on peptide quantitation. Anal. Biochem. 356, 235-243 (2006).

