Isolation and Culture of Neural Crest Stem Cells from Human Hair Follicles
Summary
This article presents a robust protocol for isolation and culture of neural crest stem cells from human hair follicles.
Abstract
Hair follicles undergo lifelong growth and hair cycle is a well-controlled process involving stem cell proliferation and quiescence. Hair bulge is a well-characterized niche for adult stem cells1. This segment of the outer root sheath contains a number of different types of stem cells, including epithelial stem cells2, melanocyte stem cells3 and neural crest like stem cells4-7. Hair follicles represent an accessible and rich source for different types of human stem cells. We and others have isolated neural crest stem cells (NCSCs) from human fetal and adult hair follicles4,5. These human stem cells are label-retaining cells and are capable of self-renewal through asymmetric cell division in vitro. They express immature neural crest cell markers but not differentiation markers. Our expression profiling study showed that they share a similar gene expression pattern with murine skin immature neural crest cells. They exhibit clonal multipotency that can give rise to myogenic, melanocytic, and neuronal cell lineages after in vitro clonal single cell culture. Differentiated cells not only acquire lineage-specific markers but also demonstrate appropriate functions in ex vivo conditions. In addition, these NCSCs show differentiation potential toward mesenchymal lineages. Differentiated neuronal cells can persist in mouse brain and retain neuronal differentiation markers. It has been shown that hair follicle derived NCSCs can help nerve regrowth, and they improve motor function in mice transplanted with these stem cells following transecting spinal cord injury8. Furthermore, peripheral nerves have been repaired with stem cell grafts9, and implantation of skin-derived precursor cells adjacent to crushed sciatic nerves has resulted in remyelination10. Therefore, the hair follicle/skin derived NCSCs have already shown promising results for regenerative therapy in preclinical models.
Somatic cell reprogramming to induced pluripotent stem (iPS) cells has shown enormous potential for regenerative medicine. However, there are still many issues with iPS cells, particularly the long term effect of oncogene/virus integration and potential tumorigenicity of pluripotent stem cells have not been adequately addressed. There are still many hurdles to be overcome before iPS cells can be used for regenerative medicine. Whereas the adult stem cells are known to be safe and they have been used clinically for many years, such as bone marrow transplant. Many patients have already benefited from the treatment. Autologous adult stem cells are still preferred cells for transplantation. Therefore, the readily accessible and expandable adult stem cells in human skin/hair follicles are a valuable source for regenerative medicine.
Protocol
1. Preparation of Tissue Culture Plates
- Coat each well with enough Poly-D-Lysine (PDL) to cover the bottom of the well. Allow the plates to dry in the hood.
- After the wells are dry, rinse with sterile water, and aspirate. Allow the plates to dry in the hood.
- When dry, coat with fibronectin (that was dissolved in biowhittaker water overnight at 37 °C at a concentration of 1 mg in 6 ml).
- Add NCSC medium [95 ml DMEM/F12, 1 ml Penn/Strep (P/S), 1 ml N2, 2 ml B27, 100 μl mercaptoethanol (2ME; 50 mM stock), bFGF (20 ng/ml medium), IGF-1 (20 ng/ml of medium) and EGF(20 ng/ml of medium)] before fibronectin dry in the plates,
2. Extract Hair Follicle Cells from Human Scalp
- Before starting the procedure of isolating NCSCs, one needs to prepare the respective medias and reagents (see Table 1).
- Fresh adult human scalp skin from facelift procedures or fetal scalp tissue is collected, washed with PBS containing Penicillin-Streptomycin.
- The skin is then transferred into 50 ml tubes and incubated in DMEM with Dispase (10 mg/ml) overnight at 4 °C. Incubation for 2-4 hr at 37 °C is also effective. Skin pieces should be a maximum width of 1 cm to allow for enzyme to penetrate.
- Transfer the skin into a sterilized Petri dish; pull off each hair from the skin by grasping the hair shaft near the skin surface and pulling firmly and smoothly. The hair follicles show morphology of either anagen (Figure 1A) or telogen (Figure 1B).
- Incubate the isolated follicle fragments in 0.05% trypsin-EDTA (Invitrogen) for 15-20 min at room temperature with shaking periodically, add 4 ml DMEM with 10% FBS to stop reaction. The follicular epithelium is trypsinized and filtered through 40 μm filter to obtain a single-cell suspension containing cells of varying size and shape.
- Spin at 200 x g for 5 min, discard supernatant carefully, re-suspend in 1 ml PBS containing 2% serum (FBS).
- Alternatively, plucked hair follicles can be placed in culture without trypsin digestion to grow hair spheres in situ.
3. Isolation of Hair Follicle NCSCs Using Flow Cytometric Cell Sorting
- Hair follicle single-cell suspension were obtained by trypsin digestion and labeled with antibodies against CD271 (APC-conjugated) /HNK1 (FITC-conjugated) or CD271/ alpha4 integrin (PE-conjugated) for 40 min on ice in the dark.
- Centrifuge for 5 min at 200 x g at room temperature and aspirate the supernatant.
- Resuspend cells in PBS containing 2% serum (FBS), and before sorting, PI is added to gate out the dead cells.
- Perform cell sorting by flow cytometry (FACS). Collect CD271+, HNK1+ double positive cells or CD271+, alpha4 integrin+ double positive cells (Figure 2).
- After sorting, CD271+, HNK1+ double positive cells or CD271+, alpha4 integrin+ double positive cells are cultured in ultra-low attachment plates in the NCSC medium
4. Culture of Primary NCSCs
- Culture the disassociated follicular cells or FACS sorted cells in ultra-low attachment plates in the NCSC medium, change medium every day.
- Check cell culture every day under microscope. NCSCs will start to form floating small aggregates after several days and well-formed spheres in 2-5 weeks in the culture depending on age of the donors (Figure 3A). Outgrowth will appear in a few days at the bulge region and well-formed spheres in situ in several weeks (Figure 3B) when entire hair follicles are cultured in the NCSC medium.
5. Expansion of NCSCs
- Wash cells with PBS once.
- Add pre-warmed (to 37 °C, critical) accutase and incubate at 37 °C for 5-10 min with shaking periodically to make sure Neural stem cell spheres dissociated into single cell( for attachment cultured NCSC, incubate cells with accutase at 37 °C for 5 min to make sure hair follicle stem cells detached).
- Wash and centrifuge cells twice for 5 min at 200 x g at room temperature in DMEM/F12 medium to remove any remaining accutase solution.
- Re-suspend cells with NCSC culture medium.
- For floating culture, put the cells in ultra-low attachment plates. For attachment culture, put the cells in pretreated tissue culture plates.
6. Representative Results
NCSC culture medium is sufficient to maintain hNCSCs in an undifferentiated state without the need for feeder cells. Keratinocytes will not proliferate and gradually died in the medium. Certain small round cells will proliferate and form small aggregates in suspension after 3 to 5 days. These floating aggregates slowly increased in size, generated three-dimensional sphere-like structures, which we termed hair spheres (Figure 3A). If cultured in coated plates, the NCSCs will attach to the surface and not form any spheres. The attached NCSCs grow faster than in suspension. The sphere-forming or attached stem cells express NCSCs markers. When entire hair follicles are cultures, spheres are formed at the area corresponding to the bulge region (Figure 3B) .
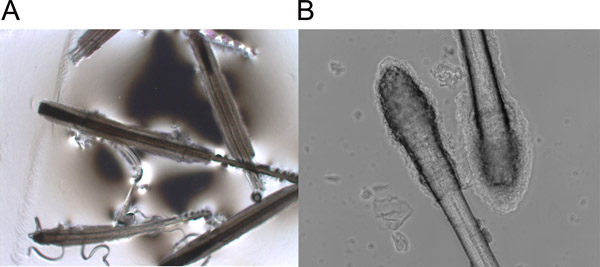
Figure 1. Plucked anagen (A) and telogen (B) hair follicles.
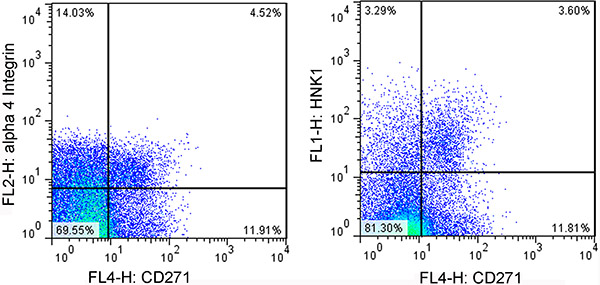
Figure 2. Representative images of FACS analysis of NCSCs. Left panel: cells are gated using anti-CD271 and anti-alphe 4 integrin antibodies. Right panel: cells are gated using anti-CD271 and anti-HNK1 antibodies.
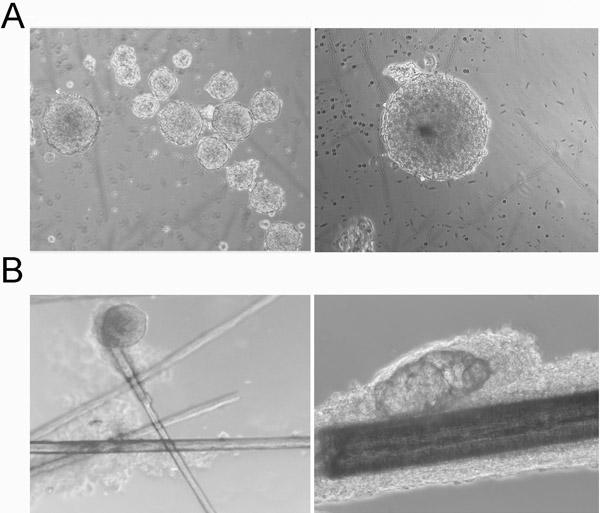
Figure 3. Morphology of hair spheres. (A) Morphology of floating hair spheres, left panel: low power, right panel: high power. (B) Morphology of hair sphere in situ at the bulge region, left panel: a hair sphere at telogen bulge, right panel: a hair sphere at the bulge region of an anagen hair follicle.
Discussion
The cell isolation and culture methods described are reproducible and robust. We have generated NCSCs from dozens of individuals across a broad age range. Although it is best to process the tissue right after tissue harvest, we found that scalp tissues can be safely stored in media on ice for overnight transportation with minimal impact on cell viability.
It is important to treat the scalp tissue with antibiotics and use aseptic technique during hair follicle isolation to avoid potential microorganism contamination. Discarded facelift skin or fetal scalp tissue yields hundreds of viable follicles and usually generate sufficient tissue for FACS sorting or further experiments in only a few days. Punch biopsy of scalp tissue usually generates limited number of cells and requires additional culture tissue to produce sufficient cells for further experiments.
Embryonic NCSCs give rise to a multitude of different cell types in the body11, it appears that hair follicle derived NCSCs also have a broad spectrum of differentiation capacity. Hair bulge is the niche for different type of stem cells3,12. Lyle and colleagues have shown isolation of human epithelial stem cells from hair bulge and these cells are multiple potent along the epithelial lineages13. Melanocyte precursors also reside in the hair bulge3. It is therefore possible to simultaneously isolate different populations of stem cells by culturing hair follicle-derived cells in mediums with different type of growth factors. Hair follicle derived stem cells are a promising source for cell regenerative therapies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is supported by NIH grant R01AR054593 and R01AR054593-S1 to Xu.
Materials
| Name of the reagent | Company | Catalogue number |
| DMEM | Invitrogen | 11965-092 |
| DMEM/F12 | Invitrogen | 11330-32 |
| Heat-inactivated FBS | Hyclone | SH30071.03 |
| B27 supplement | Invitrogen | 17504044 |
| N2 supplement | Invitrogen | 17502048 |
| bFGF | Invitrogen | PHG0026 |
| EGF | R&D system | 236-EG-01M |
| IGF-I | R&D system | 291-G1-050 |
| 0.05% Trypsin/EDTA | Invitrogen | 25300-054 |
| Dispase | Invitrogen | 17105041 |
| Penicillin-Streptomycin | Invitrogen | 15070063 |
Table 1.
References
- Cotsarelis, G., Cheng, S. Z., Dong, G., Sun, T. T., Lavker, R. M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 57, 201-209 (1989).
- Cotsarelis, G., Sun, T. T., Lavker, R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 61, 1329-1337 (1990).
- Tanimura, S., et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 8, 177-187 (2011).
- Yu, H., et al. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am. J. Pathol. 168, 1879-1888 (2006).
- Yu, H., Kumar, S. M., Kossenkov, A. V., Showe, L., Xu, X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J. Invest. Dermatol. 130, 1227-1236 (2010).
- Wong, C. E., et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J. Cell Biol. 175, 1005-1015 (2006).
- Sieber-Blum, M., Grim, M., Hu, Y. F., Szeder, V. Pluripotent neural crest stem cells in the adult hair follicle. Dev. Dyn. 231, 258-269 (2004).
- Amoh, Y., et al. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc. Natl. Acad. Sci. U.S.A. 102, 17734-17738 (2005).
- Koliatsos, V. E., Xu, L., Yan, J. Human stem cell grafts as therapies for motor neuron disease. Expert Opin. Biol. Ther. 8, 137-141 (2008).
- McKenzie, I. A., Biernaskie, J., Toma, J. G., Midha, R., Miller, F. D. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J. Neurosci. 26, 6651-6660 (2006).
- LeDouarin, N. M., Kalcheim, C. . The Neural Crest. , (1999).
- Tumbar, T., et al. Defining the epithelial stem cell niche in skin. Science. 303, 359-363 (2004).
- Roh, C., et al. Multi-potentiality of a new immortalized epithelial stem cell line derived from human hair follicles. In Vitro Cell Dev. Biol. Anim. 44, 236-244 (2008).

