Imaging Odor-Evoked Activities in the Mouse Olfactory Bulb using Optical Reflectance and Autofluorescence Signals
Summary
This article presents the protocols of intrinsic optical signals and flavoproteins autofluorescence signals imaging to map odor-evoked activities at the surface of the olfactory bulb in mice.
Abstract
In the brain, sensory stimulation activates distributed populations of neurons among functional modules which participate to the coding of the stimulus. Functional optical imaging techniques are advantageous to visualize the activation of these modules in sensory cortices with high spatial resolution. In this context, endogenous optical signals that arise from molecular mechanisms linked to neuroenergetics are valuable sources of contrast to record spatial maps of sensory stimuli over wide fields in the rodent brain.
Here, we present two techniques based on changes of endogenous optical properties of the brain tissue during activation. First the intrinsic optical signals (IOS) are produced by a local alteration in red light reflectance due to: (i) absorption by changes in blood oxygenation level and blood volume (ii) photon scattering. The use of in vivo IOS to record spatial maps started in the mid 1980’s with the observation of optical maps of whisker barrels in the rat and the orientation columns in the cat visual cortex1. IOS imaging of the surface of the rodent main olfactory bulb (OB) in response to odorants was later demonstrated by Larry Katz’s group2. The second approach relies on flavoprotein autofluorescence signals (FAS) due to changes in the redox state of these mitochondrial metabolic intermediates. More precisely, the technique is based on the green fluorescence due to oxidized state of flavoproteins when the tissue is excited with blue light. Although such signals were probably among the first fluorescent molecules recorded for the study of brain activity by the pioneer studies of Britton Chances and colleagues3, it was not until recently that they have been used for mapping of brain activation in vivo. FAS imaging was first applied to the somatosensory cortex in rodents in response to hindpaw stimulation by Katsuei Shibuki’s group4.
The olfactory system is of central importance for the survival of the vast majority of living species because it allows efficient detection and identification of chemical substances in the environment (food, predators). The OB is the first relay of olfactory information processing in the brain. It receives afferent projections from the olfactory primary sensory neurons that detect volatile odorant molecules. Each sensory neuron expresses only one type of odorant receptor and neurons carrying the same type of receptor send their nerve processes to the same well-defined microregions of ˜100μm3 constituted of discrete neuropil, the olfactory glomerulus (Fig. 1). In the last decade, IOS imaging has fostered the functional exploration of the OB5, 6, 7 which has become one of the most studied sensory structures. The mapping of OB activity with FAS imaging has not been performed yet.
Here, we show the successive steps of an efficient protocol for IOS and FAS imaging to map odor-evoked activities in the mouse OB.
Protocol
1. Preparing the animal for imaging (in accordance with European recommendations for care and use of laboratory animals, 86/609/EEC directive)
- 6 to 8 weeks old C57BL6 male mice are anesthetized with a cocktail of ketamine (10mg/kg) and xylazine (100mg/kg) injected intraperitonealy. Surgery begins when the mouse no longer responds to hindpaw pinch. During the entire experiment the animal is placed on a heating pad. Body temperature is continuously monitored and maintained at 37°C. Depth of anesthesia is maintained throughout surgery and imaging session by checking out the absence of limb withdraw. A subcutaneous injection of 20% of the initial anesthetic cocktail is otherwise administered.
- Using clippers, remove the hair from the scalp. Clean the exposed skin from residual hair by using sterile gauze soaked with saline.
- Place the mouse in the stereotaxic frame. The snout has to be in the same plane as the back of the head, in order to set the surface of the olfactory bulb to the horizontal. Secure firmly the ear and nose bars in order to prevent movements during imaging.
- Apply ophthalmic ointment on the animal’s eyes to prevent drying and pain.
- Disinfect all surgical instruments with 70° ethanol and the scalp area with successive sweeps of betadine.
- To remove the skin covering the skull, start by making an incision in the skin with scissors at the back of the head between the ears. Then cut in both sides towards the base of the ear and in the anteroposterior direction towards the forehead along the eyelids. Finish removing the scalp by cutting the skin on top of the snout close to the nose bar.
- Under binocular observation, use a cotton swab soaked with saline to gently detach the periosteum on the top of the skull. Use a pair of forceps to remove the remaining tissue and scrape the surface of the skull with a scalpel to have a clean preparation.
- The OB is a symmetric structure composed of two hemibulbs which are located between the eyelids. They are limited in the rostral and in the caudal direction by a venous sinus, and separated by the sagittal suture. Place a piece of absorbable gelatin sponge soaked with distilled water on the bone above the OB. It is important to keep this bone area moist throughout the experiment.
2. Preparing the cranial window
- Remove the gelatin sponge and start by gently scraping the bone with n°10 scalpel blade. Keep a constant angle of 45° between the blade and the bone, and move the blade from the eyelids to the sagittal side of the bulb area. Don’t apply vertical pressure on the bone or scratch the bone above the venous sinus.
- During the thinning process, stop every 5min and place a hydrated gelatin sponge on the bone to cool down the preparation. Swab the skull with the sponge to remove bone dust.
- Keep sweeping and cooling alternatively until visualizing the trabeculae, the spongy bone layer. The fine vasculature of the OB must be visible at this stage. Stop scratching the bone, and start using the scalpel’s tip perpendicularly to “draw” a rectangular area enclosing the OB. At this stage n°11 scalpel blade can be used. Keep the surgery within the limits of the venous sinuses that should be safe of any scalpel stroke.
- Dig gradually the formed rectangular trench by using successive motions of the scalpel. Wipe the tip of the scalpel periodically to clean it and keep it sharp. Be extra cautious of the depth of the tip to avoid touching the dura surface.
- In order to get a sense of the thickness of the remaining bone, push it gently with the tip of a pair of forceps. If the bone flap folds under pressure, move to the next step.
- Under a drop of saline, use the tip of the scalpel oriented horizontally to lift up the bone flap. The removal of the flap has to be done carefully to avoid tearing off the remaining bone.
- Once the OB’s surface is exposed, check for the absence of any bleeding or blood vessels anastomosis. Injuring the dura or the tissue surface will reduce the chances of obtaining optical signals. Wipe the area with a gelatin sponge soaked in saline in order to keep the bulb moist.
- Apply polyacrylate dental cement to form a well on the bone around the window.
- Place a drop of low melting point agarose (1.2%) over the dura, and put a sterile cover glass at the dimensions of the window. During the imaging session, a small quantity of agarose can be added in order to compensate the desiccation. Agarose will prevent the OB from moving with respiration and will provide a flat surface for optical imaging.
3. Optical imaging setup for olfactory activity mapping
- The olfactory stimulation must be precisely defined in time and intensity by the use of an olfactometer. We use a custom modified version of multivial perfusion system Valvebank 8II from Automate Scientific associated with a basic air compressor (compressed breathable air would be suited as well). This system allows for accurate and fast external valve control. Solutions of pure odorant are diluted in mineral oil at the chosen concentration. To activate the dorsal OB aldehydes such as hexanal are commonly used. A precise volume of the diluted odorant (20 to 50μl) is loaded on a filter paper and placed in a syringe reservoir. Through the perfusion system, pressure controlled air is delivered to the system, ensuring a constant rate of odorized air flux delivery to the animal nose during valve opening. Odorant is delivered through a Tygon R-3603 Vacuum Tubing (Saint-Gobain Corporation) carrying air at a flow rate of ˜1000ml/min. Avoid contamination between odorants and residual quantities of odorant in the tubing. If available, reproducibility of the odorant stimulus can be controlled through the use of a flame ionization detector (microFID 2020 Photovac).
- The optical setup is turned on. It consists of a cooled interlaced 12 bits CCD camera (ORCA AG Hamamatsu) associated with a fluorescence stereomicroscope (Leica MZ16), a computer controlled olfactometer and stabilized excitation lamps with appropriate bandpass interference filters. A scheme describing our setup is provided in figure 2. For Intrinsic Optical Signals Imaging (IOSI), a 200W tungsten halogen lamp (Oriel QTH) coupled with a fiber ring light (Schott) plugged around the microscope objective are used to provide stable and even illumination. For Flavoprotein Autofluorescence Signals Imaging (FASI), a 150W metal Halide lamp (Leica) with a 5mm core liquid light guide provide even localized excitation of the fluorescence through the epi-illumination port of the stereomicroscope.
Image acquisition and hardware synchronization are realized by custom software. The open source software Micromanager can also be used to control the optical setup and acquisition.
4. Optical imaging
- Place the stereotaxic frame under the stereomicroscope, the cranial window centered in the field of view (see Fig. 2A for a schematic of the optical setup).
- Tune the microscope to focus on the capillaries. Prior to stimulation trials (see description in 5) an image of the bulb is taken under 560nm (green) light (fiber ring) which provides a good contrast for blood vessels imaging. This picture is used as an anatomic control to check the state of the preparation and is acquired several times during the experiment.
- For IOS imaging, reflected light intensity is recorded by the CCD camera under 630+/-10 nm (red) illumination. Images are acquired at full frame (no binning) at 5 frames per second which correspond to an exposure time of about 150ms. Power of the light source is adjusted to reach gray levels of at least ˜3000 on the OB area, close to the saturation of the CCD pixels wells. Doing so makes it possible to take advantage of the 12bits dynamics of the CCD to capture faint intensity changes during activation. Maximum IOS amplitudes reach ˜1%.
- For FAS imaging fluorescence is acquired under excitation of 480nm+/-20nm (blue) epiflurorescence light. A high-pass filter at 515nm is set up to capture light. Images are acquired at 5 frames per second with a binning of 4 by 4 to maximize sensitivity. Excitation light power is adjusted similarly to IOS with gray levels of 3000 on the OB area. Maximum FAS amplitudes reach ˜3%.
For both imaging modalities, the depth of field in the subject plane is the same and was measured at 0.5 mm for a magnification of about 4 times. - Light should be turned off between imaging trials to avoid heating and photobleaching of the preparation.
5. Imaging trials
- Standard imaging session included a baseline of 5 to 10s where only air is delivered, followed by the odor stimulation for 3 to 10s depending on the chosen odor concentration, and 70 to 82s are further recorded with a constant air flow for baseline recovery (Fig. 2A). At the end of the trial, light is turned off and pure air is delivered for the inter trial interval duration (1 to 3 minutes) to wash out residual odor molecules and avoid sensory habituation. Odor trials were interleaved with blank trials (air delivery).
- Odor-evoked activated zones are visualized as roughly spherical areas on the maps and correspond to the size of individual glomeruli (80 to 200μm in diameter9). Image processing is explained in Figure 2B. Olfactory maps are presented in Figure 3. Contrary to any other sensory system, the signal-to-noise ratio in the OB was sufficient to resolve odor-evoked responses in single trials (Fig. 3B for IOS and Fig. 3D for FAS).
- Mice are euthanized immediately at the end of the imaging session using methods in accordance with European recommendations for care and use of laboratory animals.
6. Representative Results (see olfactory maps in figure 3):
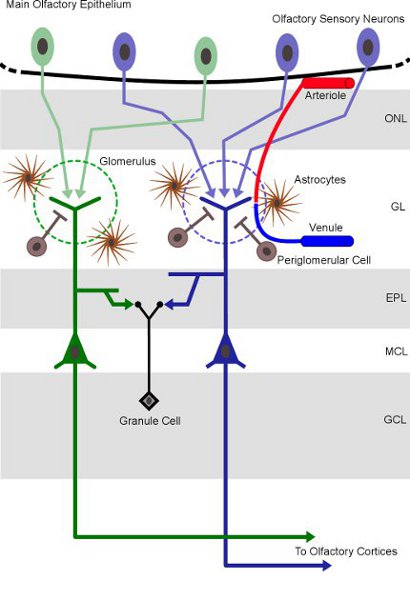
Figure 1 Structural organization of the main olfactory bulb in rodents. Olfactory sensory neurons, primary sensory cells located in the main olfactory epithelium, express the same odorant receptor and converge onto the same glomeruli in the OB. Olfactory glomeruli, the round-shaped neuropils (dashed circles), are located at the surface of the OB. Note that a very dense and complex vascular network is present at the glomerular level. Abbreviations (top/down): ONL: olfactory nerve layer; GL: glomerular layer, EPL: external plexiform layer; MCL: mitral cell layer; GCL: granule cell layer.
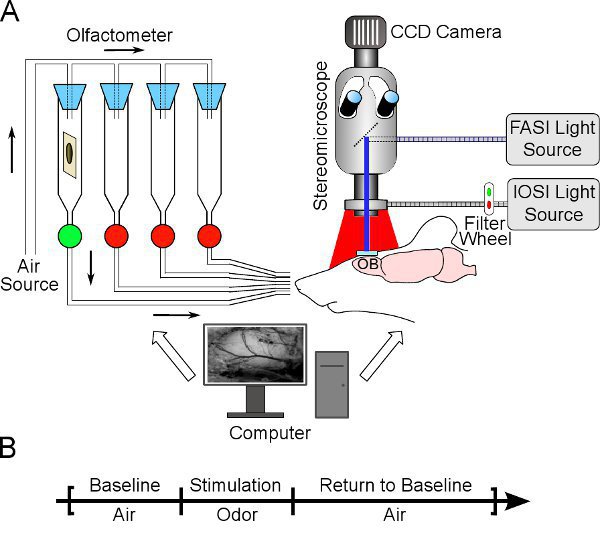
Figure 2 Reflectance and fluorescence signals recording in vivo. A. Wide-field optical imaging setup. The brain of an anesthetized mouse is exposed to either red (IOS) or blue (FAS) light through either an annular fiber ring attached to the optics lens or an epi-illumination port of a microscope. Odors are loaded into sealed vials and odorized air is delivered to the animal nose (green light: open valve). B. Recording protocol and data processing. IOS and FAS are recorded as series of individual trials (90s of duration). The diagram shows the timeline of a single trial: baseline varies from 5 to 10s, stimulation from 3 to 10s, and return to baseline from 70 to 82s. Image processing requires pixel-by-pixel subtraction of intensity values during the baseline to intensity values during periods of stimulation (for FAS) or stimulation plus return to baseline (for IOS). This difference is then divided by baseline values to obtain a variation in % (see resulting images in Fig. 3).
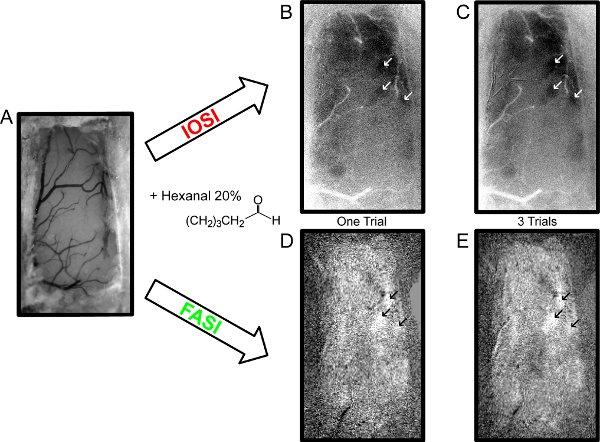
Figure 3 Odor-evoked activity maps in the OB using IOS and FAS imaging. A. Vasculature of the dorsal OB visualized under green light. B-C. IOS imaged (single trial versus three averaged trials respectively) for a 10s presentation of 20% hexanal. White arrows indicate the spherical regions of interest activated by this odor. These activation maps were obtained using the frames averaged during the first second after the end of odor stimulation (maximum of reflectance variation -0.63% in A and -0.52% in B). Note the black zones of absorbance where odor activation has occurred. C-D. FAS acquired sequentially in the same mouse for the same odorant (single trial versus three averaged trials respectively). These activation maps were obtained using the frames averaged during the first second after the beginning of odor stimulation (maximum of fluorescence variation +0.72% in D and +0.66% in E). Note that the white zones of autofluorescence emission indicated by black arrows correspond to the black zones in IOS. The grainy aspect seen in the FAS map is due to the 4 by 4 binning required for the improvement of the sensitivity. FAS images were not corrected from autofluorescence bleaching. Actual dimensions of images B-E: 0.7 mm wide x 1.2 mm long.
Discussion
In this article we present IOS and FAS imaging techniques for in vivo recordings of odor-evoked activities in the mouse OB. To achieve this goal a relatively simple and affordable wide-field optical imaging setup is necessary. The acquisition of imaging data requires training to perform the fine surgical procedures and avoid any injury to the dura or brain tissue. In particular, major bleeding will absorb photons recorded for imaging and end up the experiment.
One benefit of IOS and FAS imaging is to avoid the injection of fluorescent tracers that could result in cellular toxicity or undesired side effects. They make it possible to tackle issues about olfactory maps thus spatial coding of sensory stimuli. Contrary to 2-DeoxyGlucose imaging, they provide the possibility to image several odors in a single animal. However, since photon penetration is limited in the tissue, IOS and FAS are restricted to the dorsal part of the OB and can not be recorded from ventral regions.
Endogenous optical signal imaging offers excellent spatial resolution for in vivo imaging. Technical improvements concern quantitative calculations of vascular components in the reflectance signals8,9 as well as dynamics of blood oxygenation and volume during sensory activation10. Multiwavelength imaging of IOS imaging approaches are currently developed in our laboratory to fully quantify total hemoglobin concentration and oxygenation in the OB during sensory activation. These spectroscopic optical measurements added to FAS imaging will give the opportunity to answer the unsolved relationship between vascular and intracellular dynamics during sensory activation11,12.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the “Agence Nationale de la Recherche” grant ANR-09-JCJC-0117-01 and “Neuropôle de Recherche Francilien-NERF” grant for Romain Chery. We thank Françoise Lefebvre for the software development in C++/Qt, and Laurent Pinot and Batiste Janvier for help in the development of the optical imaging setup.
Materials
| Name of The Regent | Company | Catalogue number |
| Imalgene | Merial | |
| Rompun | Bayer | |
| Agarose, type III-A | Sigma-Aldrich | A9793-50G |
| Hexanal 98% | Aldrich | 115606-100ML |
References
- Grinvald, A., Lieke, E., Frostig, R. D., Gilbert, C. D., Wiesel, T. N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 324, 361-364 (1986).
- Rubin, B. D., Katz, L. C. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 23, 499-511 (1999).
- Chance, B., Cohen, P., J&246, b. s. i. s., &, F., Schoener, B. Intracellular oxidation-reduction states in vivo. Science. 137, 499-508 (1962).
- Shibuki, K., Hishida, R., Murakami, H., Kudoh, M., Kawaguchi, T., Watanabe, M., Watanabe, S., Kouuchi, T., Tanaka, R. Dynamic imaging of somatosensory cortical activity in the rat visualized by flavoprotein autofluorescence. J. Physiol. 549 (Pt. 3, 919-9127 (2003).
- Uchida, N., Takahashi, Y. K., Tanifuji, M., Mori, K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat. Neurosci. 3, 1035-1043 (2000).
- Gurden, H., Uchida, N., Mainen, Z. F. Sensory-evoked intrinsic optical signals in the olfactory bulb are coupled to glutamate release and uptake. Neuron. 52, 335-345 (2006).
- Soucy, E. R., Albeanu, D. F., Fantana, A. L., Murthy, V. N., Meister, M. Precision and diversity in an odor map on the olfactory bulb. Nat. Neurosci. 12, 210-220 (2009).
- Frostig, R. D., Lieke, E. E., Ts’o, D. Y., Grinvald, A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc. Natl. Acad. Sci. U.S.A. 87, 6082-6086 (1990).
- Meister, M., Bonhoeffer, T. Tuning and topography in an odor map on the rat olfactory bulb. J. Neurosci. 21, 1351-1360 (2001).
- Dunn, A. K., Devor, A., Bolay, H., Andermann, M. L., Moskowitz, M. A., Dale, A. M. Simultaneous imaging of total cerebral hemoglobin concentration, oxygenation, and blood flow during functional activation. Opt. Lett. 28, 28-30 (2003).
- L’Heureux, B., Gurden, H., Pain, F. Autofluorescence imaging of NADH and flavoproteins in the rat brain: insights from Monte Carlo simulations. Optics. Express. 17, 9477-9490 (2009).
- Renaud, R., Gurden, H., Chery, R., Bendhamane, M., Martin, C., Pain, F. Multispectral imaging of the olfactory bulb activation: influence of realistic differential pathlength correction factors on the derivation of oxygenation and total hemoglobin concentration maps (Proceedings Paper). , (2011).

