Rodent Stereotaxic Surgery and Animal Welfare Outcome Improvements for Behavioral Neuroscience
Summary
Stereotaxic surgery on rodents allows for targeted administration of drugs or electrical stimulation and recordings in awake, behaving animals. In this video presentation we will demonstrate recent procedural refinements to this long-standing procedure that successfully improved survival rate and reduced post-surgical weight loss.
Abstract
Stereotaxic surgery for the implantation of cannulae into specific brain regions has for many decades been a very successful experimental technique to investigate the effects of locally manipulated neurotransmitter and signaling pathways in awake, behaving animals. Moreover, the stereotaxic implantation of electrodes for electrophysiological stimulation and recording studies has been instrumental to our current understanding of neuroplasticity and brain networks in behaving animals. Ever-increasing knowledge about optimizing surgical techniques in rodents1-4, public awareness concerning animal welfare issues and stringent legislation (e.g., the 2010 European Union Directive on the use of laboratory animals5) prompted us to refine these surgical procedures, particularly with respect to implementing new procedures for oxygen supplementation and the continuous monitoring of blood oxygenation and heart rate levels during the surgery as well as introducing a standardized protocol for post-surgical care. Our observations indicate that these modifications resulted in an increased survival rate and an improvement in the general condition of the animals after surgery (e.g. less weight loss and a more active animal). This video presentation will show the general procedures involved in this type of stereotaxic surgery with special attention to our several modifications. We will illustrate these surgical procedures in rats, but it is also possible to perform this type of surgery in mice or other small laboratory animals by using special adaptors for the stereotaxic apparatus6.
Protocol
Note: Antiseptic techniques should be employed during the whole procedure. All the instruments and materials (cotton-tipped swabs, gauze, etc.) that will be used during the surgery should be sterilized by autoclaving. A surgical mask, hair bonnet and sterile gloves should be worn. The working area and the stereotaxic apparatus should be cleaned thoroughly, and disinfected with a 70% ethanol solution.
1. Pre-surgical procedures
- Set up the stereotaxic apparatus and all materials needed. Pre-warm the heating pad.
- Place the cannula in its support and check if it is straight.
- Turn on the gas system – mixture of ambient air and oxygen (30-35% of total flow should be oxygen).
- Weigh the rat and administer the anesthetic. We are using a mixture of ketamine (37.5 mg/kg) and dexmedetomidine (0.25 mg/kg) injected subcutaneously. For different anesthesia protocols, see Flecknell4 and Hellebrekers et al.7.
- After the rat lost consciousness, shave the head area going from the ears to just in between the eyes with an electric razor.
- Place the rat on the heating pad, with its nose in front of the air tubing. Use an oximeter to ensure that the rat has an adequate blood oxygenation level (should not drop <90%). Please follow the manufacturer’s instructions for proper use of equipment.
- Apply eye cream (Duratears Z, Alcon) on both corneas to avoid dehydration.
- Check the rat’s reflexes (tail reflex or toe-pinch reflex, as demonstrated in Walantus et al.8) to ensure that it is adequately anesthetized. If the rat continues to show strong reflexes, supplementation of anesthesia might be needed.
- If no toe-pinch reflex is shown, place the rat in the stereotaxic apparatus, adjust the ear bars so that it shows equal reading on both sides, and place again the air tubing in front of the animal by fixing it with the nose bar. Check again if the rat shows a blood oxygenation level of 90% or higher. If not, adjust either the tubing, bringing it closer to the nose, or increase the flow of oxygen. Monitor the blood oxygenation level and heart rate throughout the surgery.
- Continuously monitor the rat’s temperature with a rectal thermometer (preferentially connected to a heating pad) and record the values at the beginning and end of the surgery. Adjust the heating pad or use a blanket to maintain a body temperature of 37.5 to 38.5°C.
2. Surgery
- Inject the analgesic. We are using a single peri-operative administration of carprofen (4.0-5.0 mg/kg, subcutaneously). For different analgesic protocols, see Hellebrekers et al.7.
- Clean the shaved area of the skin from the center to the haired perimeter three times with a disinfectant (e.g., chlorhexidine 0.5%) and locally inject a mixture of lidocaine (20 mg/ml) and adrenaline (5 mg/ml) for local anesthesia and vasoconstriction (to prevent excessive bleeding).
- Make an anterior-posterior incision of about 2.5 cm on the midline of the scalp, going from between the eyes until the back of the ears. Use 4-6 bulldog clamps to pinch off the skin and to keep the incision open. Remove any conjunctive tissue with a spatula and/or cotton swabs and clean the area to expose the skull surface.
- Check if the head is level: First, find Lambda and place the guide cannula exactly over this location, touching the skull. Record the dorso-ventral coordinate. Next, place the guide cannula exactly over Bregma, touching the skull, and record its dorso-ventral coordinate. These two coordinates should be identical. If the difference is >0.3 mm, adjust the nose bar to correct it.
- Make two small holes for fixing the skull screws using a sterilized hand drill (one approximately 5 mm anterior to the cannula location in one of the hemispheres and the other 5 mm posterior to the cannula location in the contralateral hemisphere). Place two sterile screws into these holes until they are tightly anchored, without being inserted completely into the skull.
- With the guide cannula placed exactly at Bregma, record the anterior-posterior and lateral coordinates. The correct location of guide cannula placement for each brain region can be calculated by adding or subtracting from Bregma, with the aid of a stereotaxic atlas9-11.
- Position the guide cannula in its correct location, slightly touching the skull. Record the dorso-ventral coordinate. For bilateral cannula placement, find next the cannula location in the other hemisphere, and again record the dorso-ventral coordinate. Both coordinates should be identical (or differ <0.3 mm).
- Mark the cannula locations on the skull with a sterile pencil and, with the hand driller, make the burr holes, checking the size and the correct location with the aid of the guide cannula. Once the holes are made, use a sterile needle to gently punch the meninges to allow for unobstructed insertion of the cannula.
- Place the cannula into the first hole and lower it carefully until it reaches the final ventral coordinate. Prepare the dental cement and generously apply it around the cannula and one or both screws in order to fix the cannula. Wait until the cement has dried completely. Afterwards, carefully remove the cannula support by turning the dorso-ventral bar upwards.
- Place the second cannula into the support and go to the cannula location in the other hemisphere. Place the cannula into the hole and repeat the previous step. Cover the screws and a large surface of the cannulas with the cement, and before the cement is dry, remove any surplus from the skin.
- Inject warm (~37°C) sterile saline (~10 ml/kg, s.c.) to ensure rehydration.
- After the cement has completely dried, remove the cannula support and place a sterile pin into each cannula to prevent obstruction.
- Clean the wound area with sterile saline and suture the front and the back of the wound.
- Remove the animal from the stereotaxic apparatus, replacing the gas tubing in front of its nose. Continue to monitor the oxygen saturation level and body temperature.
- If an injectable anesthetic with dexmedetomidine is used, inject its antagonist atipamezole (0.25 mg/kg, s.c.) and wait until the animal wakes up (approximately 5 minutes).
- Place the rat into a recovery cage. To avoid hypothermia, place the cage in an incubator at 28°C or on a heating pad in a place where you can observe the animal for at least one hour, before returning it to the vivarium room.
3. Post-surgical care
- During the first 4 days after surgery, monitor the rat’s recovery by keeping daily records of weight and other observations concerning the condition of the animal in laboratory logbooks or “animal welfare diaries”.
- Animals that show overt signs of sickness, infection of the wound, loss of body weight or other signs of reduced well-being must undergo special care: e.g. an extra dose of analgesics to minimize post-operative pain, a mixture of powder food and water in addition to standard chow to stimulate the rat’s appetite, and/or a subcutaneous injection of saline to support rehydration.
- If the rat does not show any improvement after these interventions, or the loss of body weight is >15% (compared to pre-surgery weight), sacrifice the animal with an overdose of anesthetic (humane end-point).
- Rats usually need to recover for at least 7 days before commencement of behavioral experiments.
4. Representative Results
To determine whether the various modifications to our surgical procedure, particularly with respect to oxygen supplementation and the continuous monitoring of blood oxygenation levels, heart rate and body temperature, enhanced the animal’s survival and improved its general condition after surgery, we compared the non-survival rate of 20 cohorts of animals (consisting of 20 rats each) that underwent surgery after we implemented these modifications with the non-survival rate of 24 cohorts (20 rats each) operated with the standard protocol. As is shown in Figure 1a, the non-survival rate was significantly reduced in the sample of cohorts that was operated with the modified protocol (P < 0.05; Mann-Whitney U test, two tailed). Moreover, as is shown in Figure 1b, post-surgical weight loss of rats operated with the modified protocol was also significantly reduced as compared to that of rats operated with the standard procedure (post-operative day 1: P < 0.05; post-operative day 2: P < 0.01; post-operative day 3: P = 0.17; Student t-tests).
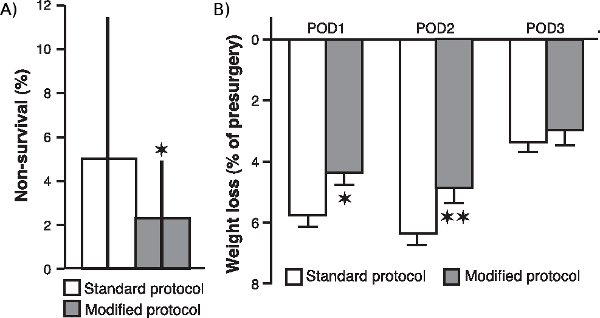
Figure 1. Effect of surgical modifications on non-survival rate and post-surgical weight loss. (A.) Non-survival rate of rats operated with the modified protocol as compared to that of rats operated with the standard protocol. The non-survival rate (median ± interquartile ranges) was calculated as the percentage of rats, per cohort of 20 rats, that did not survive surgery. *p < 0.05, Mann-Whitney U test two tailed (n = 20 cohorts for the modified protocol and 24 cohorts for the standard protocol). (B.) Weight loss (mean ± SEM as percentage of pre-surgery weight) during the first (POD1), second (POD2) and third (POD3) post-operative day. *P < 0.05, **P < 0.01, Student t-test (n = 60 per group).
Discussion
The main purpose of this video presentation is to familiarize behavioral neuroscientists with the basic principles of stereotaxic surgery. Researchers who are already performing stereotaxic surgery might also benefit from this video and consider some of the procedural refinements for use in their own laboratory. An ever-increasing knowledge about optimizing surgical techniques1-3, the development of new anesthetics and analgesics for use in human and veterinary medicine4,12, public awareness concerning animal welfare issues and stringent legislation (e.g., the 2010 European Union Directive on the use of laboratory animals5) prompted us to implement new procedures for oxygen supplementation and the continuous monitoring of blood oxygenation and heart rate levels during the surgery. We observed, as shown for a representative sample of animals, an overall increased survival rate and a significantly reduced post-surgical weight loss. Such a reduced post-surgical weight loss might reflect a smaller burden of the surgical procedure on the animal and, consequently, result in a more active animal in the immediate aftermath of surgery. Whether it also has beneficial effects on its long-term health is not clear. However, a remarkable observation was that removing the oxygen supply temporarily led to a marked and reliable decrease in blood oxygen saturation levels, which could drop even below 50% (See the video presentation for a demonstration hereof). It is conceivable that prolonged inadequate blood levels of oxygen, possibly as occurring in animals not provided with any oxygen supplementation during surgery, might result in hypoxia with long-term behavioral consequences and also negatively impact the outcome and/or quality of behavioral experimentation. We do not know whether such a depression of oxygen blood supply during surgery is specific to the anesthetic protocol used in our laboratory (i.e., a mixture of ketamine and dexmedetomidine) or whether it is a more general phenomenon associated with injectable anesthetics. The use of inhalation anesthesia, with a mixture of ambient air and oxygen, might be an alternative method to overcome the depression of blood oxygen levels.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Dr. Peter Gerrits for the drawings used in the video.
Materials
| Name of the reagent | Company |
| Alcohol 70% | VWR |
| Antisedan (atipamezole) | Orion |
| Atropine sulfate 0,5 | Pharmachemie BV |
| Bulldog haemostatic clamp | Aesculap |
| Cannulas | Component Supply Co. |
| Chlorhexidine 0.5% | AppepPharma |
| Cleaning powder | Alconox |
| Cotton sticks | Hartmann BV |
| Dexdomitor (dexmedetomidine) | Orion |
| Drill | Dremel 8000 |
| Duratears Z | Alcon |
| Durogrip Naaldvoerder converse 130mm | Aesculap |
| Durotip Fijne schaar ret.sp/st 110 mm | Aesculap |
| Gauze | Medicomp (5×5) |
| Heating pad | Harvard Apparatus |
| Insect pins (stylets) | Entosphinx (Czech Republik) |
| Ketamine 10% (ketamine) | Alfasan |
| Micro-halsted straight tip | Aesculap-vet |
| Molinea | Hartmann BV |
| NaCl 0,9% | Baxter |
| Needles (25G) | Terumo |
| Oximeter | Edan Instruments, Inc. |
| Pentobarbital | Pharmacy of the UMCG |
| Rimadyl | Pfizer |
| Scalpel blade No. 23 | Swann Morton |
| Scalpelholder NR. 4 133 mm | Aesculap |
| Screw driver | conventional Hardware Store |
| Simplex Rapid (dental cement) | Kemdent |
| Skull screws | Component Supply Co. |
| Spatula | VWR |
| Spongestan special | Johnson & Johnson |
| Stereotacts | Kopf Instruments |
| Surgical forceps 100mm | Aesculap |
| Suture material Safil 5/0 | Aesculap |
| Syringe 10 ml | Omnifix |
| Syringe 1ml | Terumo |
| Syringe 5ml | Omnifix |
| Xylocaine (lidocaine/adrenaline) | Astra Zeneca |
References
- Russell, W. M. S., Burch, R. L. . The Principles of Humane Experimental Technique. , (1959).
- Richardson, C. A., Flecknell, P. A. Anaesthesia and post-operative analgesia following experimental surgery in laboratory rodents: are we making progress?. Altern. Lab Anim. 33, 119-127 (2005).
- Stokes, E. L., Flecknell, P. A., Richardson, C. A. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim. 43, 149-154 (2009).
- Flecknell, P. A. . Laboratory Animal Anaesthesia – A Practical Introduction for Research Workers and Technicians. , (2009).
- . DIRECTIVE 2010/63/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union. , (2010).
- Geiger, B. M., Frank, L. E., Caldera-Siu, A. D., Pothos, E. N. Survivable Stereotaxic Surgery in Rodents. J. Vis. Exp. (20), e880-e880 (2008).
- Hellebrekers, L. J., Booij, L. H. D. J., Flecknell, P. A., Van Zutphen, L. F. M., Baumans, V., Beynen, A. C. Anaesthesia, analgesia and euthanasia. Principles of Laboratory Animal Science. , 277-311 (2001).
- Walantus, W., Elias, L., Kriegstein, A. In utero intraventricular injection and electroporation of E16 rat embryos. J. Vis. Exp. (6), e236-e236 (2007).
- Paxinos, G., Watson, C. . The Rat Brain in Stereotaxic Coordinates. , (1986).
- Paxinos, G., Watson, C. . The Rat Brain in Stereotaxic Coordinates. , (2007).
- Swanson, L. W. . Brain Maps: Structure of the Rat Brain. , (1992).

