Real-time Imaging of Heterotypic Platelet-neutrophil Interactions on the Activated Endothelium During Vascular Inflammation and Thrombus Formation in Live Mice
Summary
Here we report an experimental technique of fluorescence intravital microscopy to visualize heterotypic platelet-neutrophil interactions on the activated endothelium during vascular inflammation and thrombus formation in live mice. This microscopic technology will be valuable to study the molecular mechanism of vascular disease and to test pharmacologic agents under pathophysiological conditions.
Abstract
Interaction of activated platelets and leukocytes (mainly neutrophils) on the activated endothelium mediates thrombosis and vascular inflammation.1,2 During thrombus formation at the site of arteriolar injury, platelets adherent to the activated endothelium and subendothelial matrix proteins support neutrophil rolling and adhesion.3 Conversely, under venular inflammatory conditions, neutrophils adherent to the activated endothelium can support adhesion and accumulation of circulating platelets. Heterotypic platelet-neutrophil aggregation requires sequential processes by the specific receptor-counter receptor interactions between cells.4 It is known that activated endothelial cells release adhesion molecules such as von Willebrand factor, thereby initiating platelet adhesion and accumulation under high shear conditions.5 Also, activated endothelial cells support neutrophil rolling and adhesion by expressing selectins and intercellular adhesion molecule-1 (ICAM-1), respectively, under low shear conditions.4 Platelet P-selectin interacts with neutrophils through P-selectin glycoprotein ligand-1 (PSGL-1), thereby inducing activation of neutrophil β2 integrins and firm adhesion between two cell types. Despite the advances in in vitro experiments in which heterotypic platelet-neutrophil interactions are determined in whole blood or isolated cells,6,7 those studies cannot manipulate oxidant stress conditions during vascular disease. In this report, using fluorescently-labeled, specific antibodies against a mouse platelet and neutrophil marker, we describe a detailed intravital microscopic protocol to monitor heterotypic interactions of platelets and neutrophils on the activated endothelium during TNF-α-induced inflammation or following laser-induced injury in cremaster muscle microvessels of live mice.
Protocol
1. Preparation of Intravital Microscope (Figure 1A)
- Prepare superfusion buffer (125 mM NaCl, 4.5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, and 17 mM NaHCO3, pH 7.4).
- Turn on a circulatory water bath to maintain temperature of buffer and a thermo-controlled blanket at 37 °C. Aerate buffer with nitrogen gas (5% CO2 balanced with nitrogen).
- Turn on the microscope system (Sutter Lambda DG-4 high speed wavelength changer, workstation computer, Olympus BX61W microscope, MPC-200 multi-manipulator, ROE-200 stage controller, high speed camera, and intensifier).
- For the vascular inflammation model, use a 100% dichroic mirror. To induce thrombus formation by laser injury, replace a 100% mirror with a 50/50% mirror and turn on a micropoint laser ablation system.
2. Preparation of Cremaster Muscle for Intravital Microscopy (Figure 1B)
- Anesthetize a male mouse (6-8 weeks old, C57BL/6) by i.p. injection of ketamine (125 mg/kg body weight (BW)) and xylazine (12.5 mg/kg BW). The University of Illinois Institutional Animal Care and Use Committee approved all animal care and experimental procedures. For a vascular inflammation model, inject murine TNF-α (0.5 μg in 250 μl saline) intrascrotally into a mouse 3 hr prior to imaging (2-2.5 hr prior to surgery).
- Place the mouse on a thermo-controlled blanket at 37 °C on an intravital microscope tray.
- Pull up the skin on the surface of the neck using forceps and incise horizontally the midline of the neck skin up to 1 cm with scissors.
- Gently open the cervical muscle using blunt scissors and remove the muscle surrounding the trachea.
- Cannulate a PE90 tube into trachea to eliminate breathing difficulty.
- Gently remove the left side of cervical muscle and isolate the jugular vein from surrounding tissues.
- Cannulate a PE10 tube into the left jugular vein for infusion of antibodies and additional anesthetics.
- Gently pull out and incise the scrotal skin horizontally. To maintain tissue integrity throughout the experiment, prewarmed buffer was superfused over the surgical field.
- Press on the lower abdomen with one forcep and carefully pull out a testicle with the other forcep.
- Remove the surrounding connective tissues around the testis.
- Incise the cremaster muscle vertically and flatten the muscle over a glass coverslip on an intravital microscope tray by pinning the periphery.
- Allow 10-20 min for the muscle to stabilize prior to data collection. Cremaster muscle arterioles (for thrombus formation) and venules (for vascular inflammation) with a diameter of 30-45 μm were chosen for our study.
- To maintain anesthetic conditions, infuse 30-50 ml of the same anesthetic approximately every 30 min through the jugular cannulus.
3. Intravital Microscopy for TNF-α-induced Vascular Inflammation
- After placement of the mouse on the intravital microscope, open SlideBook 5.0 software and click on “Focus Window” on menu bar to set the appropriate optics and objective (60X).
- Infuse Dylight 488-conjugated rat anti-mouse CD42c (0.1 μg/g BW in 100 μl saline) and Alexa Fluor 647-conjugated rat anti-mouse Gr-1 antibodies (0.05 μg/g BW in 100 μl saline) through a jugular cannulus.
- Go to menu bar, and click on “Image Capture” in the toolbar.
- A new menu will pop up; In “Image”, select “Bin Factor” as 1×1 and select “640” on width and “460” on height.
- In “Filter Set”, mark the checkbox for the channels that you would like to expose and set the exposure time for each. For example, open channel: 10 msec, FITC channel: 50 msec, and Cy5 channel: 50 msec.
- Select “Time-Lapse Capture” to adjust number of time points and interval. For the inflammation model, the number of time points is set to 1,000-1,500 with an interval of 200 msec (Total elapsed time: 5 min).
- Click “Test” to see a single-plane image at the specified exposure time for that channel. Adjust exposure time and/or microscope light until the histogram shows an adequate intensity distribution (For multi-channel capture, repeat this process for each channel).
- Once all of the parameters are set (channels, exposure times, and so on) in the “Image Capture”, select “Start” to begin capture.
- Monitor rolling and adherent platelets and neutrophils for 5 min in the top half of an inflamed cremaster venule in the vascular inflammation model. Eight to ten different venules are recorded in one mouse.
- Images were taken using a total magnification of 60X (1.0 NA water immersion objective) giving a window size of 157 μm x 118 μm.
- Upon completion of the experiment, the mice were euthanized via cervical dislocation.
4. Data Analysis for the Venular Inflammation Model
- Open SlideBook 5.0 software and click “Folder” on the menu bar to open a file.
- Click on the drop-down channel menu and select “Open, FITC, Cy5, etc.”
- Click “Renormalize (red and green bars)” and select the channel. Drag the red and green bars to the left or right to roughly optimize the fluorescence signal.
- Click “Apply” and “OK” to update the image.
- Click “Thumbnail Button” to select current display as the default.
- Play captured timelapse image, and count rolling and adherent neutrophils during the 5 min period.
- To analyze platelet thrombus formation, go to “Mask” and select “Create”.
- Name a mask and mark “In the current image”.
- Click “Large pencil icon” in “Marquee Tool” in the main view.
- Color a region outside the vessel in the main view to set a background mask.
- Go to “Mask”, click “Copy This Plane”, click “Copy mask in current timepoint” and select “All timepoints”, and click “OK”.
- To calculate background signal, go to “Statistics” and click “Mask Statistics”.
- Click “Current 2D Time Lapse or 4D Image(s)” in Image Scope, and select “Entire Mask” in Mask Scope. In Features, select “Elapsed Time (hh:mm:ms)” under Date of Capture, select “Area (pixels)” under Morphometry, select “Maximum Intensity” under Intensity, and click “Export” (This statistic method allows us to calculate auto-fluorescence intensity (background signal)).
- Open the text file in MS Office Excel and calculate the average value of background signal throughout the recording period. This is the background fluorescence intensity.
- To determine thrombus intensity, click “Large pencil icon” again in the “Marquee Tool” in the main view.
- Color inside the vessel in the main view to set platelet signal.
- Go to “Mask”, click “Copy This Plane”, click “Copy mask in current timepoint”, select “All timepoints”, and click “OK”.
- Go to “Statistics” and click “Mask Statistics”.
- Click “Current 2D Time Lapse or 4D Image(s)” in Image Scope, and select “Entire Mask” in Mask Scope. In Features, select “Elapsed Time (hh:mm:ms)” under Date of Capture, select “Area (pixels)” under Morphometry, select “Sum Intensity” under Intensity, and click “Export” (This statistic method allow us to calculate the fluorescence intensity inside the vessel).
- Open the text file in MS Office Excel. Fluorescence signal of platelets is calculated by subtracting the average value of background intensity x Area (pixel) from the sum intensity of the inside of the vessel. This is the fluorescence intensity of the platelet thrombus.
- Repeat this in 30 different venules in 3-5 different mice. Then, calculate median value of the fluorescence intensity of platelets.
- To analyze the neutrophil rolling and adhesion, the rolling influx of neutrophils was determined over 5 min in each venule and presented as cell numbers per minute. The number of adherent neutrophils that were stationary for >30 sec and slowly crawled (<10 μm over 30 sec) but did not roll over, were counted and presented as cell numbers per 5 min.
5. Intravital Microscopy for Laser-induced Arteriolar Thrombosis
5-1 Calibration of ablation laser
- Place a mirrored slide on microscope stage, apply a small drop of distilled water to the top of the slide, and adjust the intensity and focus using the eyepiece.
- Open “Focus Window” and select the “FRAP” tab in SlideBook 5.0 software.
- Set the laser power at 40-50, and set both “Double click repetitions” and “Double click size” to 8. Mark on the “Guide” box to bring up a set of crosshairs.
- Select the “Arrow” icon from the taskbar on the top of the screen.
- Under the “FRAP Alignment” tab, click “Fire next”. A small spot should appear near the lower left corner on the monitor. Once the spot appears, double click on the center of it. Once clicked, another spot should appear above and to the right of the first spot; double click on it. Repeat this for 16 points (the 16th spot will appear in the lower right corner.) Click “Save” when complete to update the calibration parameters.
- To test the accuracy of the calibration, click on the “Center” button-a small spot should appear in the center of the screen. If not, repeat the calibration until desired accuracy is achieved.
5-2 Laser-induced arteriolar thrombus formation
- Place an anesthetized mouse (see the procedure of 2-2 to 2-12) on the microscope stage.
- Under the “Capture” window, select a protocol or create a new one. The settings are as follows: CY5 (70 msec exposure), Open (10 msec exposure), and FITC (50 msec exposure). Image is captured over 20 min (approximately 2,400 time points with an interval time of 500 msec).
- Infuse Dylight 488-conjugated anti-mouse CD42c and Alexa Fluor 647-conjugated anti-mouse Gr-1 antibodies as described above.
- Optimize microscope parameters including fluorescence intensity and bright field image as described in 3-3 to 3-7.
- Click the “Test” button to ensure proper brightfield intensity or antibody signal, arteriole placement and focus.
- Click “Start” to initiate the image capture process.
- Before firing the ablation laser, make sure that the mouse pointer icon has been selected and that the vessel wall is in clear focus.
- To fire the laser, double click on a spot 2-3 μm internally from the vessel wall. Injury of the arteriolar endothelial cells causes a visual shape change that should be apparent in the brightfield image, followed by platelet accumulation. Monitor thrombus formation for 5 min after laser injury.
- Five minutes after laser injury (the thrombus size should now be stable) pause and cancel the capture. Subsequently, start a capture with 2400 time points with a 500 msec interval to record adherent platelets and rolling/adherent neutrophils for 20 min. Three to five arterioles are recorded in one mouse.
- Upon completion of the experiment, the mice were euthanized via cervical dislocation.
6. Data Analysis for the Arteriolar Thrombosis Model
- Go over steps 4.1 through 4.5.
- To obtain the background fluorescence intensity during platelet thrombus formation, go over steps 4.7 through 4.14.
- Go to “Mask”, click “Segment”, select FITC in channel, and insert the average value of background signal on Low. Click “Apply” and “OK”.
- To analyze the fluorescence intensity of platelet thrombus (Dylight 488-conjugated anti-CD42c antibody), go over steps 4.18 through 4.19. To calculate the antibody signal, “Sum Intensity” is subtracted by the background signal (“Maximum Intensity” x “Area (pixels)”). This is the fluorescence intensity of the platelet thrombus.
- To quantify the rolling and adherent neutrophils, play the timelapse and count the number of cells that visibly roll over the platelet thrombus over 20 min. Rolling is defined as a decrease in neutrophil speed while interacting with the platelet thrombus for at least 2 sec. Adherent neutrophils are defined as any neutrophils that remain attached to the platelet thrombus for at least 2 min. The number of rolling and adherent neutrophils was presented as cell numbers per 20 min. Rolling velocity was calculated using a particle tracking system.
Representative Results
Using a detailed intravital microscopy analysis, heterotypic platelet-neutrophil interactions on the activated endothelium were visualized by infusion of fluorescently-labeled antibodies against a platelet (CD42c) or neutrophil marker (Gr-1) into live mice.
In a model of TNF-α-induced venular inflammation, most rolling neutrophils were stably adhered to the endothelium presumably by interaction of activated β2 integrins with ICAM-1 during the recording period (3-4.5 hr after injection of TNF-α, Figure 2A).8 Rolling and adherent neutrophils were already present before the video capture began due to the TNF-α-induced endothelial cell activation. The number of rolling and adherent neutrophils on the inflamed endothelial cells was 0.25 ± 0.05 cells per minute and 18.5 ± 0.7 cells per 5 min, respectively (Figures 2B and 2C). Most neutrophils were adhered to the activated endothelium for the duration of video capture (1-1.5 hr), and there was only a minimal decrease in adherent cells along with a minimal increase in rolling cells (data not shown). We found that most platelets adhere to adherent and crawling neutrophils rather than the inflamed vessel wall (Figure 2A, Video 1). Platelet thrombi accumulated and embolized repeatedly for the duration of the video capture. The integrated fluorescence signal associated with adherent platelets was shown in Figure 2D.
Utilizing the laser-induced arteriolar thrombosis model, we were able to examine and characterize the heterotypic interaction of platelets and neutrophils at the site of arteriolar wall injury. In this model, the thrombus size peaked around 100 sec after laser injury, followed by a series of rapid small embolization for the next 2-3 min (data not shown).9,10 Five minutes after laser injury, the size of platelet thrombus remained relatively constant during imaging (5-25 min after laser injury, Figures 3A-3C) and neutrophils rolled on and adhered to the platelet thrombus (Figures 3A-3B, Video 2). The fluorescence signal from the circulating platelets was negligible in comparison with that from the platelet thrombus. The number of rolling and adherent neutrophils was 21.5 ± 3.0 and 1.6 ± 0.4 cells over 20 min, respectively (Figures 3D-3E). Initial rapid rolling of neutrophils occurred on the endothelial cells. Once neutrophils contacted the platelet thrombus, the rolling velocity of neutrophils on a platelet thrombus was changed with a range of 8.2 ± 1.1 μm per second (Figure 3F), which is mediated by interaction of P-selectin and PSGL-1.11
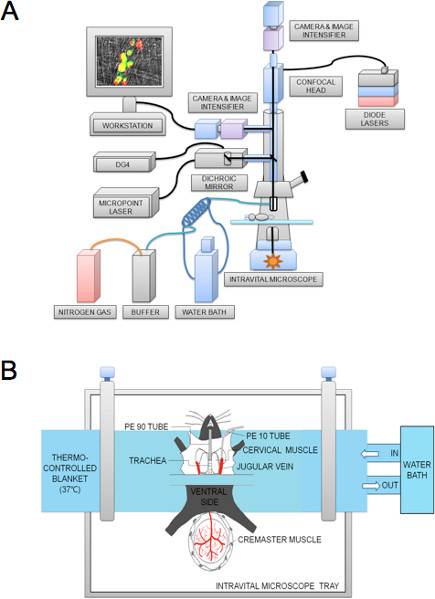
Figure 1. Schematic of the intravital microscope system (A) and preparation of the cremaster muscle microvessel (B).
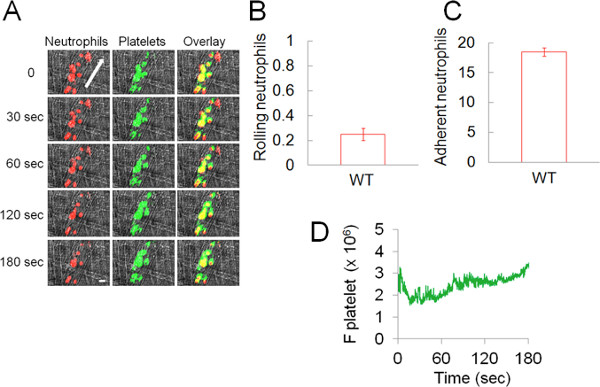
Figure 2. Heterotypic interactions of platelets and neutrophils during the TNF-α-induced venular inflammation in live mice. Platelets and neutrophils were detected by Dylight 488-conjugated anti-mouse CD42c and Alexa Fluor 647-conjugated anti-mouse Gr-1 antibodies, respectively. (A) Representative binarized images of the appearance of fluorescence signals associated with neutrophils (red) and platelets (green) over 180 sec. Arrow shows direction of blood flow. Bar = 10 μm. (B-C) The number of rolling (cells/minute) and adherent neutrophils (cells/5 min) on inflamed endothelial cells is shown. Data represent the mean ± SEM of the 30 different venules in 4 wild-type mice. (D) Median integrated fluorescence signal of platelets (F platelet) is plotted as a function of time. No signal was detected with Dylight 488-conjugated control rat IgG (data not shown).
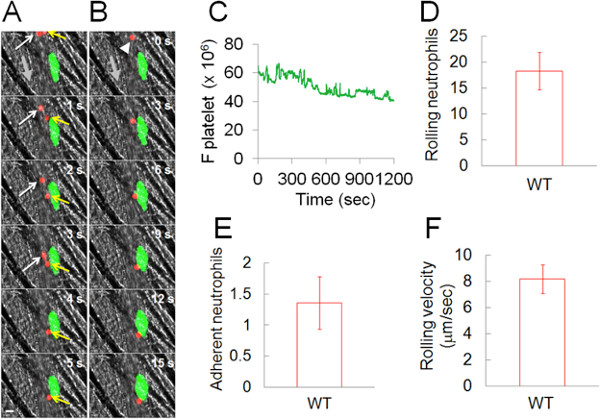
Figure 3. Heterotypic interaction of neutrophils with a platelet thrombus at the site of laser-induced arteriolar injury in live mice. Platelets and neutrophils were detected as described in Figure 2. (A) A single neutrophil (red, a yellow arrow) rolls over the platelet thrombus (green) while a second neutrophil (red, a white arrow) rapidly rolls over arteriolar endothelial cells, shown over 5 sec. (B) A single neutrophil (red) rolling over and adhering to a platelet thrombus (green) shown over 15 sec. The arrowhead shows rolling and adherent neutrophils. Bar = 10 μm. The thick, grey arrow shows the direction of blood flow. (C) Median integrated fluorescence signal of platelets (F platelet) is plotted as a function of time. (D-E) The number of rolling and adherent neutrophils on the platelet thrombus is shown (cells/20 min). (F) The rolling velocity of neutrophils over the platelet thrombus. Data represent the mean ± SEM of the 14 thrombi in 5 wild-type mice.
Discussion
Here we describe a detailed protocol for real-time fluorescence intravital microscopy to visualize heterotypic platelet-neutrophil interactions on the activated endothelium during vascular inflammation and thrombosis. Previously, similar fluorescence microscopic approaches were reported to study the molecular mechanism of thrombus formation and vascular inflammation.8,12 Since the heterotypic cell-cell interaction could be important for vaso-occlusion at the injury site, this technology will be a valuable tool for studying the cellular and molecular mechanisms of vascular disease. The advantage of real-time imaging technology is to monitor immediate and subsequent interactions of intravascular cells on the activated/damaged endothelium under inflammatory and thrombotic conditions. Further, our microscopic system could be utilized in studying heterotypic interactions between circulating tumor cells and endothelial or blood cells using fluorescently-labeled tumor cells or antibodies against tumor cell markers such as human epithelial cell adhesion molecule (EpCAM).13,14 Instead of using fluorescently-labeled antibodies, this microscopy could also be performed with transgenic mice expressing fluorescent proteins in a specific cell type such as CD41-EYFP+ megakaryocytes.15
Using the intensity of fluorescently-labeled antibodies, we could determine the kinetics of platelet thrombus formation and molecular expression on activated intravascular cells following vessel injury. However, most antibodies used in this purpose inhibit the antigen function which may result in unexpected outcomes. Therefore, the experiment should be carried out with special care to optimize the concentration at which the antibody gives the sufficient fluorescence signal by its binding to the antigen without affecting the antigen function. In addition, the antibody specificity is another issue. It is known that subpopulations of dendritic cells and monocytes express low levels of Gr-1.16
Nevertheless, we found that monocytes are only 3-5% of rolling leukocytes during acute inflammation and thrombus formation, as determined by a fluorescently-labeled antibody against mouse F4/80 that is expressed exclusively in monocytes and dendritic cells (data not shown). Therefore, most rolling and adherent leukocytes during TNF-α-induced venular inflammation and laser-induced arteriolar thrombosis would be neutrophils.
In this report, we used two mouse models of vascular disease. Although we understand that no animal model can recapitulate human disease conditions, the work carried out in microvessels of live mice using this technology will have a direct relevance to human disease and will be easily translatable to better treatment of thrombo-inflammatory diseases.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by grants from National Institutes of Health (P30 HL101302 and RO1 HL109439 to J.C.) and American Heart Association (SDG 5270005 to J.C.). A. Barazia was supported by a T32HL007829 NIH training grant.
Materials
| Name of Reagent/Material | Company | Catalog Number | Comments |
| NaCl | Fisher Scientific | 7647-14-5 | |
| KCl | Sigma-Aldrich | 7447-40-7 | |
| CaCl2 2H2O | Sigma-Aldrich | 10035-04-8 | |
| MgCl2 6H2O | Fisher Scientific | 7791-18-6 | |
| NaHCO3 | Fisher Scientific | 144-55-8 | |
| 0.9% NaCl Saline | Hospira | 0409-4888-10 | |
| Ketamine | Hospira | 0409-2051-05 | |
| Xylazine | Lloyd | ||
| Intramedic Tubing (PE 90) | BD Diagnostics | 427421 | |
| Intramedic Tubing (PE 10) | BD Diagnostics | 427401 | |
| Murine TNF-α | R&D Systems | 410-MT | |
| Dylight 488- labeled rat anti-mouse CD42b antibody | Emfret Analytics | X488 | |
| Alexa Fluor 647-conjugated anti-mouse Ly-6G/Ly-6C (Gr-1) Antibody | BioLegend | 108418 | |
| NESLAB EX water bath/circulator | Thermo-Scientific | ||
| Olympus BX61W microscope | Olympus | ||
| TH4-100 Power | Olympus | ||
| Lambda DG-4 | Sutter | ||
| MPC-200 multi-manipulator | Sutter | ||
| ROE-200 stage controller | Sutter | ||
| C9300 high-speed camera | Hamamatsu | ||
| Intensifier | Video Scope International | ||
| Ablation Laser | Photonic Instruments, Inc. | ||
| SlideBook 5.0 | Intelligent Imaging Innovations |
References
- Wagner, D. D., Frenette, P. S. The vessel wall and its interactions. Blood. 111, 5271-5281 (2008).
- Nieswandt, B., Kleinschnitz, C., Stoll, G. Ischaemic stroke: a thrombo-inflammatory disease. J. Physiol. 589, 4115-4123 (2011).
- Yang, J., Furie, B. C., Furie, B. The biology of P-selectin glycoprotein ligand-1: its role as a selectin counterreceptor in leukocyte-endothelial and leukocyte-platelet interaction. Thromb. Haemost. 81, 1-7 (1999).
- Zarbock, A., Polanowska-Grabowska, R. K., Ley, K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 21, 99-111 (2007).
- Chen, J., Lopez, J. A. Interactions of platelets with subendothelium and endothelium. Microcirculation. 12, 235-246 (2005).
- Konstantopoulos, K., et al. Venous levels of shear support neutrophil-platelet adhesion and neutrophil aggregation in blood via P-selectin and beta2-integrin. Circulation. 98, 873-882 (1998).
- Maugeri, N., de Gaetano, G., Barbanti, M., Donati, M. B., Cerletti, C. Prevention of platelet-polymorphonuclear leukocyte interactions: new clues to the antithrombotic properties of parnaparin, a low molecular weight heparin. Haematologica. 90, 833-839 (2005).
- Hidalgo, A., et al. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat. Med. 15, 384-391 (2009).
- Cho, J., Furie, B. C., Coughlin, S. R., Furie, B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J. Clin. Invest. 118, 1123-1131 (2008).
- Cho, J., et al. Protein disulfide isomerase capture during thrombus formation in vivo depends on the presence of beta3 integrins. Blood. 120, 647-655 (2012).
- Gross, P. L., Furie, B. C., Merrill-Skoloff, G., Chou, J., Furie, B. Leukocyte-versus microparticle-mediated tissue factor transfer during arteriolar thrombus development. Journal of Leukocyte Biology. 78, 1318-1326 (2005).
- Falati, S., Gross, P., Merrill-Skoloff, G., Furie, B. C., Furie, B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 8, 1175-1181 (2002).
- Barthel, S. R., et al. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proceedings of the National Academy of Sciences of the United States of America. 106, 19491-19496 (2009).
- Trzpis, M., McLaughlin, P. M., de Leij, L. M., Harmsen, M. C. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. The American Journal of Pathology. 171, 386-395 (2007).
- Junt, T., et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 317, 1767-1770 (2007).
- Egan, C. E., Sukhumavasi, W., Bierly, A. L., Denkers, E. Y. Understanding the multiple functions of Gr-1(+) cell subpopulations during microbial infection. Immunologic Research. 40, 35-48 (2008).

