Dissection and Lateral Mounting of Zebrafish Embryos: Analysis of Spinal Cord Development
Summary
Developmental processes such as proliferation, patterning, differentiation, and axon guidance can be readily modeled in the zebrafish spinal cord. In this article, we describe a mounting procedure for zebrafish embryos, which optimizes visualization of these events.
Abstract
The zebrafish spinal cord is an effective investigative model for nervous system research for several reasons. First, genetic, transgenic and gene knockdown approaches can be utilized to examine the molecular mechanisms underlying nervous system development. Second, large clutches of developmentally synchronized embryos provide large experimental sample sizes. Third, the optical clarity of the zebrafish embryo permits researchers to visualize progenitor, glial, and neuronal populations. Although zebrafish embryos are transparent, specimen thickness can impede effective microscopic visualization. One reason for this is the tandem development of the spinal cord and overlying somite tissue. Another reason is the large yolk ball, which is still present during periods of early neurogenesis. In this article, we demonstrate microdissection and removal of the yolk in fixed embryos, which allows microscopic visualization while preserving surrounding somite tissue. We also demonstrate semipermanent mounting of zebrafish embryos. This permits observation of neurodevelopment in the dorso-ventral and anterior-posterior axes, as it preserves the three-dimensionality of the tissue.
Introduction
Visualization of the spinal cord in zebrafish is inhibited by a number of factors. Due to the thickness of the overlying somites and the internal location of the spinal cord, a considerably long working distance is required for high cellular resolution. The yolk ball (which is still present during early stages of neurogenesis) further increases the working distance required, and is easily damaged by pressure from a coverslip. In addition, debris from damaged yolk prohibits clear visualization of tissues. Though cross sections in the dorso-ventral (DV) axis are possible, they do not readily permit simultaneous visualization in the anterior-posterior (AP) axis1.
To overcome these obstacles, embryos are dissected and mounted on slides. This procedure provides several benefits. First, zebrafish embryos readily lie with the lateral side facing upwards, which facilitates AP axis visualization. Second, removal of the yolk ball decreases the required working distance, and limits debris. Third, this mounting procedure allows for both fluorescent and brightfield microscopy. Fourth, mounted embryos are stable for months at 4 °C, allowing prolonged specimen visualization. Finally, progression of development occurs in that anterior segments are more mature than posterior segments. In order to ensure that staged matched spinal hemisegments are compared between embryos, overlying somite tissue is used as a guide. For example, the tenth most posterior somite overlies the tenth spinal hemisegment, which is developmentally equivalent in stage-matched embryos. This mounting procedure of intact embryos allows easy identification of somites.
We use genetics and gene knock-down technologies to study the mechanisms of axon guidance in the developing spinal cord. In particular, we have determined that robo2, robo3, and dcc are required for Commissural Primary Ascending (CoPA) axon pathfinding. Using the mounting procedure described here, we were able to examine ventral growth, midline crossing, commissure width, dorsal and anterior growth2,3. This mounting procedure may also be applied to DV or AP patterning of the spinal cord. We have used this procedure to determine disparate roles of downstream Wnt effectors in DV patterning of the spinal cord. Using this mounting, we were able to obtain resolution of DV markers at the single cell level, and determine minute patterning shifts as a result of altered Wnt signal reception4,5. This mounting procedure also allows for mitotic index calculation through anti-phosphohistone 3 or BrdU labeling (progenitor proliferation) differentiation5.
Protocol
1. Mounting Embryos (Upon Completion of Selected Visualization Technique, such as Immunocytochemistry, in situ Hybridization, etc.)
- Place embryos in a 35 mm Petri dish filled with buffer of choice (Figure 1A). The choice of buffer is based on the labeling procedure. Typically PBS-based buffers are suitable.
- Under a dissecting microscope, and using forceps, secure the head with one pair of forceps while pulling the yolk away with the other pair (Figure 1B). Alternatively, use an insect pin in an insect pin holder to gently pull away the yolk.
- Using the embryo poker (fishing line (0.41 mm diameter) glued to either a capillary tube or a Pasteur pipette) move the embryos to a part of the Petri dish that does not have a lot of yolk debris (Figures 1C-D).
- Using a glass Pasteur pipette, aspirate the embryos in as little liquid as possible, and gently pipette them onto a slide (Figure 1E).
- Gently wick away excess liquid using a laboratory wipe. Avoid contact with embryos.
- Add one drop of mounting medium to embryos. For fluorescent labels, use an antifade reagent of choice. For colorimetric signals, 70% glycerol may be used as a mounting medium.
- Using the embryo poker, orient the embryos on their sides in rows (Figure 1F).
- Add one "dab" of petroleum jelly or high vacuum grease to each corner of a coverslip (Figure 1G). This prevents damage of embryo from excessive compression.
- Place coverslip (petroleum jelly side down) on top of embryos (Figure 1H).
- Gently tap the coverslip on each corner until the mounting media (70% glycerol or anti-fade mounting reagent) is touching the coverslip (Figure 1H).
- If necessary, add more mounting media by pipetting right next to the coverslip, using a pipettor in the range of 20-200 ml. It will wick under.
- Using a laboratory wipe, completely clean around the coverslip. Be certain to not apply pressure to the coverslip, as this will damage embryos. This area must be dry and have no petroleum jelly or mounting media on it.
- Use nail polish to seal around the edge of the coverslip (Figure 1I).
Representative Results
When elucidating the mechanisms that underlie various developmental events such as cellular patterning, differentiation, and axon guidance, it is important to be able to visualize cells within the context of their tissue. Dorsoventral patterning defects typically present as a ventral or dorsal shift in the expression domain of transcription factors in the pax, nkx, or dbx families4. At times, changes may be subtle, comprising only a few cell diameters4. Cross sectional analysis permits this type of analysis. However, reagents with weaker signals, or cross sections that are not perpendicular to the AP axis can limit interpretation. Further, cross sectional analysis does not readily take into account whether changes persist in the AP axis, and relies on the availability of a cryostat. This limitation is circumvented by lateral views of the spinal cord. As seen in Figure 2B, nkx6.1 mRNA is distributed in roughly the ventral half of the spinal cord at 24 hr post-fertilization (hpf), within the progenitor domain. Progenitor cells are distinguishable from post mitotic cells due to presence of the floor plate, which is found in the medial part of the spinal cord. Viewed with DIC optics, individual cells are clearly distinguishable, and a defined boundary between expressing and nonexpressing cells is evident.
Progenitor cell cycle exit is accompanied by changes in gene expression. For example, all post-mitotic neurons express HuC/D which is detectable with immunocytochemistry1,5. mRNA probes for islet, gata, and vsx families label specific post-mitotic neurons of consistent position and number within each spinal cord hemisegment6. In addition, axon guidance receptors such as those in the robo family are expressed in restricted populations of postmitotic neurons (Figure 2C)2,7,8. Lateral mounting of embryos allows accurate counts of post-mitotic neurons. Similarly, progenitors that continue to divide can be quantified with anti-phospho-histone 3 immunocytochemistry as well as BrdU labeling. Furthermore, cell death can be assessed with TUNEL labeling4-6.
At 24 hpf, axons of the following postmitotic neurons can be visualized with various methods, and are distinguishable at the single cell level: Dorsal Lateral Ascending (DoLA), Commissural Primary Ascending (CoPA), Commissural Secondary Ascending (CoSA), Ventral Longitudinal Descending (VeLD), Kolmer-Agdur (KA), Commissural Bifurcating/Longitudinal (CoB/L), Circumferential Ascending (CiA), Circumferential Descending (CiD), and Unipolar Commissural Descending (UCoD), Rohon-Beard (R-B), motoneurons (M)9. Axons extend from these neurons in the dorsal, ventral, anterior, and posterior directions. They branch, cross the midline, and exit both the dorsal and ventral spinal cord. When coupled with confocal laser scanning microscopy, these varied cellular behaviors are evident in laterally mounted embryos, using immunocytochemistry and genetically encoded fluorescent proteins such as GFP2. In Figure 2D, znp-1 immunocytochemistry was used to label developing motoneurons. Motoneurons exit the spinal cord ventrally to innervate the surrounding developing musculature.
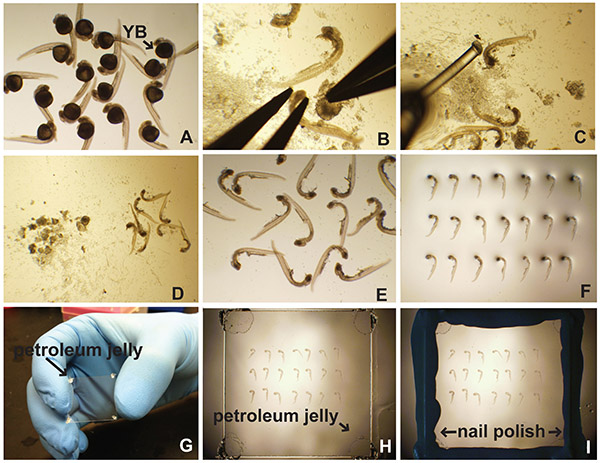
Figure 1. Dissection and lateral mount of zebrafish embryos. (A) After processing for immunofluorescence, in situ hybridization, etc., embryos are placed in a 35 mm Petri dish filled with PBT (PBS with 0.5% Triton X-100) or PTw (PBS with 0.1% Tween-20). The yolk ball (YB) is evident. (B) The yolk is removed by securing the head of the embryo followed by careful dissection. (C) An embryo poker (fishing line glued to a Pasteur pipette) is used to separate embryos and yolk debris (D). Cleaned embryos are pipetted onto a microscope slide (E) and aligned (F). (G) Petroleum jelly is applied to the corners of the coverslip. (H) The coverslip is placed gently on top of the embryos in mounting medium. (I) Nail polish is used to seal the edges of the coverslip. Click here to view larger image.
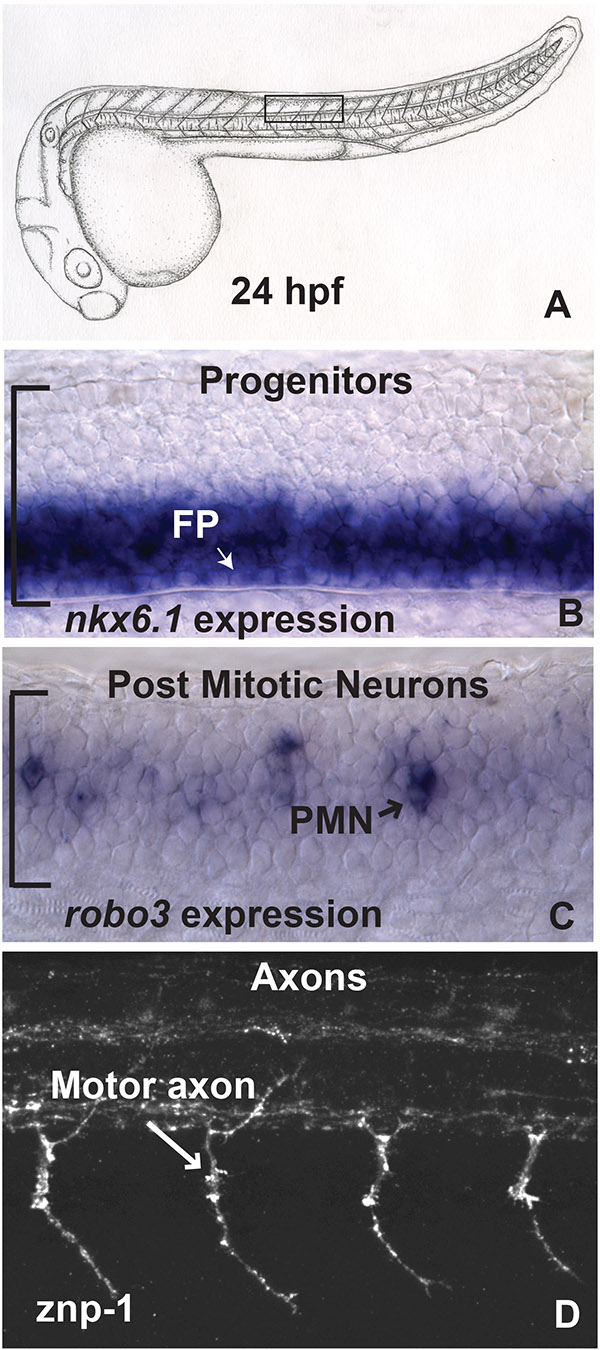
Figure 2. Analysis of gene expression patterns and axon pathfinding in laterally mounted zebrafish embryos. (A) Drawing of a zebrafish embryo at 24 hpf. In B-D, roughly four hemisegments above the yolk sac extension are visualized (boxed area). (B) nkx6.1 is expressed in the ventral spinal cord, including the floor plate (FP). (C) robo3 is expressed in postmitotic neurons (PMN). The floor plate and progenitor zone is not evident in this more lateral focal plane. In B,C, the spinal cord is bounded by a bracket on the left hand side of the image. (D) Confocal microscopy was used to image znp-1 immunofluorescence, which labels motor axons exiting the spinal cord ventrally. In all images, anterior is to the left and dorsal is up. A 40X long working distance, water immersion (N.A. 0.8) lens was used (3.3 mm). Click here to view larger image.
Discussion
Lateral mounting of fixed zebrafish embryos aids visualization of spinal cord development. Through removal of the yolk ball, the working distance required for exceptional microscopic imaging is reduced. Our representative results were limited to the 24 hpf stage, however, this technique can be used as early as 18 hpf, though earlier embryos are more difficult to dissect due to the size of the embryo and the fragility of the tissue. This technique is also applicable to embryos older than 24 hpf. Aided by the optical clarity of the embryonic zebrafish, the ability to perform forward genetic screens, reverse genetics, and transgenic approaches, several developmental processes may be investigated. This includes mitotic indices of neuronal progenitors with markers like BrdU and anti-phospho-histone 3 4-6 and patterning through expression of neuronal and glial progenitor markers in the pax, nkx, dbx, and olig families1,4-6. Reagents that report glial and neuronal determination (such as glial fibrillary acid protein (GFAP) and HuC/D)1,4,5,10 can be used. Neuronal subtype differentiation can be analyzed through expression of islet, vsx, engrailed, and gata genes1,4-6. And finally, analysis of axon guidance is possible through antibodies such as anti-acetylated tubulin, 3A10, and znp-1 2,11,12. Though other vertebrate model systems (mouse and chick) are used to study spinal cord development, only the zebrafish permits simultaneous viewing of all developmental axes in the intact embryo.
The obvious limitation to this approach is that the embryos are fixed, which precludes live imaging. In fact, removal of (or damage to) the yolk results in rapid embryonic death, thus it is not possible to reduce the working distance in live embryos using this technique. However, high magnification spinal cord imaging in live embryos is possible. In order to preserve the yolk during live imaging, embryos can be placed in depression slides, which provide a larger space between the specimen and the coverslip. Alternatively, several 22 mm x 22 mm coverslips can be glued on either slide of a 3 in x 1 in microscopic slide. The coverslips serve as a "bridge" for the overlying coverslip. If embryos are older than 18 hpf, tricaine is used to anesthetize embryos to prevent movement. While a working distance of 0.288 mm is required for deyolked embryos at 24 hpf, when working with live embryos embedded in agarose, with intact yolks, a microscopic lens with a working distance of 3.3 mm is sufficient. Alternatively, explant cultures derived from deyolked embryos may be visualized using a plasma clot immobilization technique13. Various populations of cells can be observed through the use of genetically encoded fluorescent proteins such as GFP, mCherry, or the photoconvertible dye Kaede (to name a few). Further, fluorescently labeled cells within chimeric embryos can also be viewed with this approach14.
A consistent mounting technique that is stable for months is a critical tool for developmental and cell biologists. This straightforward technique is reproducible, allowing easy comparison between different experimental trials. Furthermore, the alignment of embryos in rows readily permits identification of specific embryos for later analysis.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Skidmore Faculty Development Grant funded the preparation and publication of this manuscript.
Materials
|
Petri dishes 35mm X 10mm |
VWR |
25373-041 |
|
Dumont Forceps #3 |
Fisher Scientific |
NC9839169 |
|
Cover glass |
Fisher Scientific |
12-541-B22X22-1.5 |
|
Slides |
Fisher Scientific |
12-550-343 |
|
SlowFade Gold |
Fisher Scientific |
S36936 |
|
ProLong Gold |
Life Technologies |
P36934 |
|
Petroleum Jelly |
Any grocery store |
|
|
Loop Holders |
VWR |
80094-482 |
|
Insect Pins |
Fine Science Tools |
26002-10 |
|
Nickel Plated Pin Holders |
Fine Science Tools |
26016-12
|
|
Name of Equipment |
Company |
Catalog number |
|
Olympus Stereo microscope |
Olympus |
SZ61 |
References
- Gribble, S. L., Nikolaus, O. B., Dorsky, R. I. Regulation and function of Dbx genes in the zebrafish spinal. Dev. Dyn. 236 (12), 3472-3483 (2007).
- Bonner, J., et al. Midline crossing is not required for subsequent pathfinding decisions in commissural neurons. Neural Dev. 7 (1), 18 (2012).
- Ross, A. B. J. Activation of Wnt signaling using Lithium Chloride: Inquiry-Based Undergraduate Laboratory Exercises. Zebrafish. , (2012).
- Bonner, J., et al. Proliferation and patterning are mediated independently in the dorsal spinal cord downstream of canonical Wnt signaling. Dev. Biol. 313 (1), 398-407 (2008).
- Gribble, S. L., et al. Tcf3 inhibits spinal cord neurogenesis by regulating sox4a expression. Development. 136 (5), 781-789 (2009).
- England, S., et al. Roles of Hedgehog pathway components and retinoic acid signalling in specifying zebrafish ventral spinal cord neurons. Development. 138 (23), 5121-5134 (2011).
- Challa, A. K., Beattie, C. E., Seeger, M. A. Identification and characterization of roundabout orthologs in zebrafish. Mech Dev. (1-2), 101-101 (2001).
- Lee, J. S., Ray, R., Chien, C. B. Cloning and expression of three zebrafish roundabout homologs suggest roles in axon guidance and cell. 221 (2), 216-230 (2001).
- Downes, G. B., Waterbury, J. A., Granato, M. Rapid in vivo labeling of identified zebrafish neurons. Genesis. 34 (3), 196-202 (2002).
- Kim, H., et al. Notch-regulated oligodendrocyte specification from radial glia in the spinal cord of zebrafish embryos. Dev. Dyn. 237 (8), 2081-2089 (2008).
- Sylvain, N. J., Brewster, D. L., Ali, D. W. Zebrafish embryos exposed to alcohol undergo abnormal development of motor neurons and muscle fibers. Neurotoxicol. Teratol. 32 (4), 472-480 (2010).
- de Soysa, T. Y., et al. Macondo crude oil from the Deepwater Horizon oil spill disrupts specific developmental processes during zebrafish embryogenesis. BMC Biol. 10 (40), (2012).
- Langenberg, T., Brand, M., Cooper, M. S. Imaging brain development and organogenesis in zebrafish using immobilized embryonic explants. Dev Dyn. 228 (3), 464-474 (2003).
- Deschene, E. R., Barresi, M. J. Tissue Targeted Embryonic Chimeras: Zebrafish Gastrula Cell Transplantation. J. Vis. Exp. (31), (2009).

