Single-cell Microinjection for Cell Communication Analysis
Summary
We describe here how to perform a single-cell microinjection of Lucifer Yellow to visualize cellular communication via gap-junctions in living cells, and provide some useful tips. We expect that this paper will help everyone to evaluate the degree of cellular coupling due to functional gap junctions. Everything described here could be, in principle, adapted to other fluorescent dyes with molecular weight below 1,000 Daltons.
Abstract
Gap junctions are intercellular channels that allow the communication of neighboring cells. This communication depends on the contribution of a hemichannel by each neighboring cell to form the gap junction. In mammalian cells, the hemichannel is formed by six connexins, monomers with four transmembrane domains and a C and N terminal within the cytoplasm. Gap junctions permit the exchange of ions, second messengers, and small metabolites. In addition, they have important roles in many forms of cellular communication within physiological processes such as synaptic transmission, heart contraction, cell growth and differentiation. We detail how to perform a single-cell microinjection of Lucifer Yellow to visualize cellular communication via gap-junctions in living cells. It is expected that in functional gap junctions, the dye will diffuse from the loaded cell to the connected cells. It is a very useful technique to study gap junctions since you can evaluate the diffusion of the fluorescence in real time. We discuss how to prepare the cells and the micropipette, how to use a micromanipulator and inject a low molecular weight fluorescent dye in an epithelial cell line.
Introduction
Gap junctions are intercellular channels that allow the intercommunication among neighboring cells1. This communication connects two or more neighboring cells, where each one contributes with a connexon or hemichannel to form the intercellular channel. In mammalian cells, the connexon is formed by six connexins, monomers with four transmembrane domains and a C and N terminal within the cytoplasm2. Gap junctions not only permit the flow of ions, second messengers and small metabolites, but also contribute to many forms of cellular communication in many physiological processes, such as synaptic transmission, heart contraction, cell growth and differentiation3,4,5,6,7,8. In addition gap junctions have been associated with many diseases including cancer9,10, muscular atrophy11, some genetic diseases and demyelinating diseases12.
This type of intercellular crosstalk can be evaluated by several methods13,14,15,16. In this paper, we show how to perform a single-cell microinjection of Lucifer Yellow to visualize cellular communication via gap-junctions in living cells. We discuss how to prepare the cells and the micropipette, the usage of the micromanipulator and the injection of Lucifer Yellow dye in a thymic epithelial cell line. Usually, this experimental procedure could be analyzed by the average of connected cells to the cell loaded with dye. In addition, this method could be used with other fluorescent dyes with molecular weight below the gap junctions cut-off which is approximately 1,000 daltons.
Protocol
1. Preparation of Cells
- Maintain a culture of a thymic epithelial cell line (IT76M1) or cell to be tested in an incubator (37°C/5% CO2).
- Wash the cells with PBS 1x (repeat this item 3x).
- Add Trypsin to the cells for 5 min.
- Add medium (twice of the volume of trypsin added in item 1.3) with 10% FBS (fetal bovine serum) to the cells with trypsin and centrifuge (800 x g for 5 min).
- Count the cells in a hemocytometer.
- Adjust the density of cells according to the cell type as the cells have to be in close contact with each other to allow coupling. Note: In our case, we used 3 x 105 cells per 35 mm Petri dish.
2. Micropipette Preparation
- Pull the micropipette as specified from a glass capillary micropipette (1.5 mm diameter) to a final 0.2 µm of diameter so as to attain a final resistance of approximately 30 MΩ17,18.
NOTE: Alternatively, injection pipettes can be purchased. The resistance depends on the cell size, for instance a higher resistance microelectrode would be necessary for pancreatic acinar cells, for example (100-150 MΩ)19. A common problem that could occur is the precipitation of Lucifer Yellow solution which can then obstruct the micropipette and may require prior filtration or centrifugation. Before injection, the micropipette should be analyzed under the microscope to detect if there is an obstruction or any type of disruption13. The micropipette can be tested by injecting LY with the micropipette tip inside a saline solution.
3. Testing the Micropipette
- Prepare the Lucifer Yellow solution (5%) in 150 mmol/L LiCI and load the micropipette using a syringe or by backfilling (put into it LY Solution).
- Place the micropipette over the 35 mm Petri dish with the IT76M1 cells on the microinjection workstation and submerge the tip of the glass micropipette into the cell medium. Focus on the micropipette and perform a dye flowing test by applying a pulse.
4. Single-cell Lucifer Yellow Microinjection
- Focus the microscope right above the cell layer using a high magnification (40X), then slowly lower the pipette to the cells using the micromanipulator.
- Puncture the target cell when the tip is close enough to touch the cell membrane, and apply a small hyperpolarizing pulse to introduce the LY into the cell. The applied voltage will depend on the net charge of dye to be injected. Alternatively, some other dyes could be used with this technique as shown in Table 1.
Note: In principle any hydrophilic dye with MW less than 1KDa could be used. However, the rate of transfer could vary according to the weight and hydrophilicity. Additionally, unspecific transfer of the dye used must be evaluated. - Capture cell images 3 min after dye injection or make a small movie with time lapse microscopy (30 fps).
NOTE: A similar approach could be seen in Hitomi et al (2015)20. To avoid communication by intercellular bridges (incomplete mitosis), a co-injection of rhodamin dextran (from 2 to 10 KDa), which does not pass through gap junctions but passes through intercellular bridges and certain types of nanotubules is recommended as shown in Figure 2.
Representative Results
Thymic epithelial cell line IT-76MI were used to evaluate dye coupling by gap junctions as these cells were described to express functional gap junctions formed by connexin 4321. Figure 1 shows the injection of Lucifer Yellow when applied in the one cell below the tip of the pipette. After few minutes, connected cells become fluorescent (asterisks) indicating the diffusion of the fluorescent dye through the gap junctions. The number of cells and time to became fluorescent is directly associated with the degree of cellular communication among these cells. Figure 2 shows the injection of LY in thymic epithelial cells and the inserts (insert d) show the co-injection of LY and Rhodamine Dextran (10KDa). As expected the LY passes to a neighboring cell, although the Rhodamine Dextran does not because of its high molecular weight. In addition, the presence of a GJ blocker (insert f), octanol, blocked the passage of the dye to the surrounding cells. Alternatively, one can evaluate the degree of coupling of a specific cell or the effect of a drug on the function of GJ. Figure 3A shows the injection of LY in TEC cells with or without dexamethasone. Then 100 cells were injected, in the presence of dexamethasone and the percentage of 1 or 2 cells communicating was similar in relation to the control without the drug. However the number of 3 or 4 cells communicating was higher when compared to control. Five or 6 cells and 7 or 8 cells with GJ communication in the presence of dexamethasone was not observed in the control, thus indicating that dexamethasone increased the degree of coupling in TEC cells.
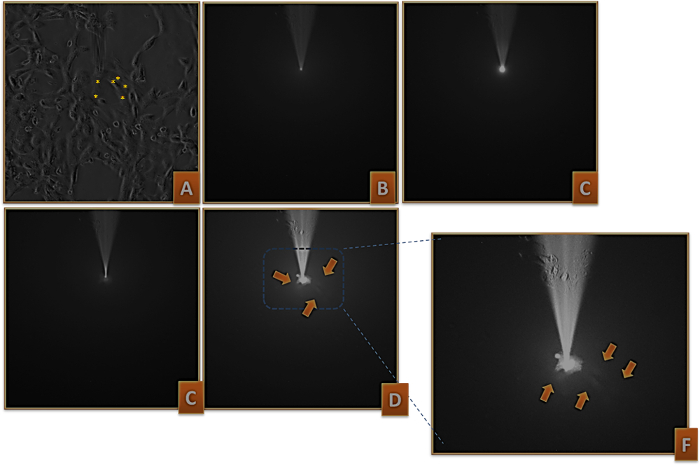
Figure 1: Lucifer Yellow injection in IT-76MI cells. (A) The micropipette close to the cell membrane. (B) The pipette is seen by fluorescence microscopy. (C) A test pulse was generated to verify whether the electrode is actually injecting the dye. (D) The pipette touched the cell membrane and it was charged with Lucifer Yellow dye. (E) The cell charged allowed the dye to pass through gap junctions to at least five neighbor cells (indicated by the arrows), X20. F) A digital zoom was done to allow better visualization. Asterisks point out the cells that were charged in the contrast phase microscopy (Figure 1A). Please click here to view a larger version of this figure.
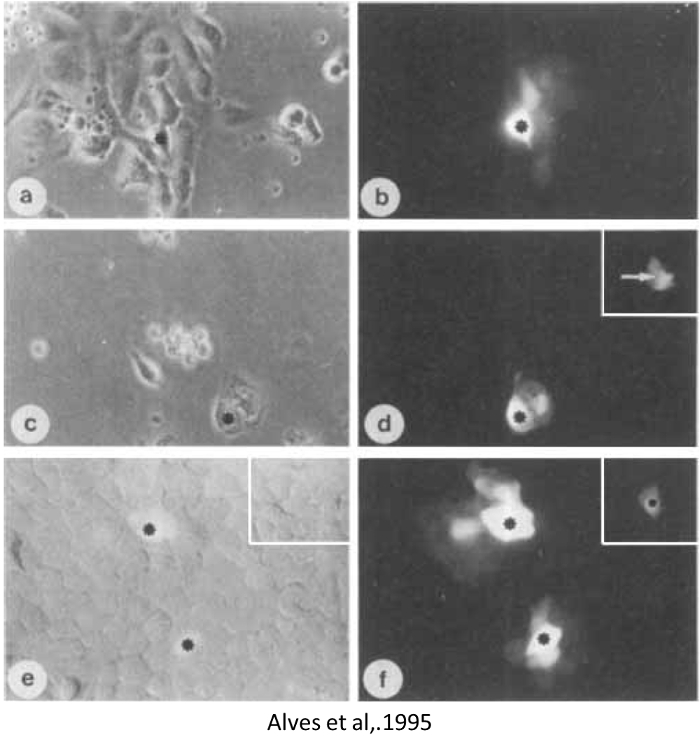
Figure 2: Lucifer Yellow injection in thymic epithelial cells (TEC). Phase contrast and fluorescence microscopy. (A and B) Human Thymic Epithelial cell. (C and D) Thymic Nurse Cell, (E and F) A Mouse Thymic Epithelial cell line, respectively. The insert in (D) shows the same cell (arrow) after an injection of rhodamine-dextran 10KDa (not permeable through gap junctions). The inserts in (E) and (F) show the absence of dye transfer in the TEC line pre-treated with octanol 1 mM (a gap junction blocker) for 10 min. In all panels asterisks mark the injected cells. (A-F) X 200. Reproduced from Alves et al., 19955. Please click here to view a larger version of this figure.
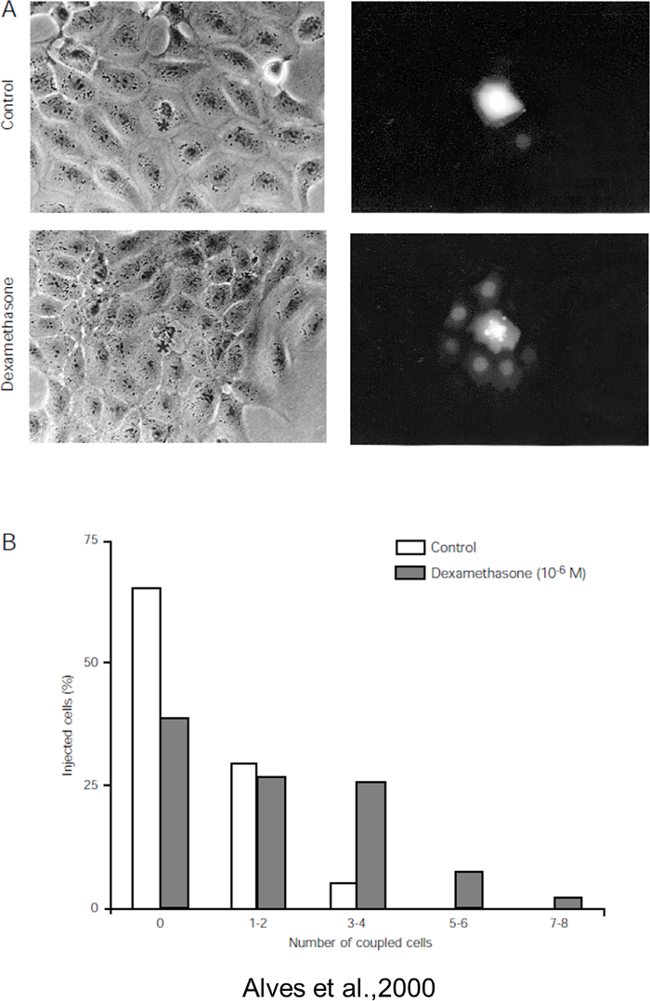
Figure 3: Gap junction communication increased by dexamethasone in a rat epithelial cell line. TEC were treated with 1 µM dexamethasone, and coupling degree was evaluated by the Lucifer Yellow dye transfer assay. (A) Microscopy fields (phase contrast and fluorescence, respectively, in the left and right panels) depicting the injected cell and those that were coupled when LY was injected (magnification 320X). In all panels asterisks mark the injected cells. (B) Histograms showing the pattern of coupling of control and dexamethasone-treated cells. The analysis comprises of 100 microinjections per group. Reproduced from Alves et al., 200023. Please click here to view a larger version of this figure.
| DYE | MW | Excitation/Emission |
| hydroxycoumarin carboxylic acid | 206 | 386/488 |
| calcein blue | 321 | 360/449 |
| 4′,6-diamidino-2-phenylindole dihydrochloride | 279 | 358/461 |
| carboxyfluorescein | 376 | 492/517 |
| ethidium iodide(bromide) | 314 | 518/605 |
| Lucifer Yellow CH | 443 | 428/536 |
| alexa fluor 488 | 570.5 | 495/519 |
| calcein | 622 | 494/517 |
| propidium iodide | 414 | 535/617 |
Table 1: Dyes currently used for microinjection experiments. Here in, some used dyes with molecular weight and the excitation/emission wavelength.
Discussion
In order to verify the presence of functional intercellular gap junction, the use of tracers, which are membrane impermeable, although permeable by intercellular channels are required16. Fluorescein, the first fluorescent dye to observe cell-to-cell coupling22, is permeable between non junctional membranes3 and has therefore been substituted by Lucifer Yellow dye15. Currently, to find the best choice among the many different types of florescent tracers depends on the scope and conditions of the experiment. The procedure of cell loading with fluorescent dyes permits the evaluation of morphology, function of single cells and the kinetic rate of transfer between cells. Furthermore, dye microinjection allows a better understanding of the physiological role of gap junctions between cells21,23, since the degree of cellular communication is related with the number of coupled cells.
Several key factors are crucial for successfully obtaining microinjection data. Normal cell homeostasis and integrity must be maintained, thus a short injection time (<1 s) of dye and technical expertise with microinjection is needed. Key factors in getting a better resolution of the captured images is having a cooled CCD camera, the fluorescence filters in place and the use of a high NA objective14. Also, some tips could be useful as: 1) cell culture dishes with grids are recommended to visualize a particular injected cell, also glass-bottom dishes should be used for high-resolution microscopy applications; 2) it is recommended to make 10-20 pulled needles before starting microinjections; 3) depending on the resistance of the electrode tip can be very helpful, loading the micropipette with the dye using capillarity by touching the pipette tip into the dye solution; 4) it is not necessary to load the whole body of the pipette, a few microliters to fill the tip and 2 to 3 mm above the tip is sufficient; 5) start the experiment with a less magnified lens and try to see the shadow of the pipette walls. Afterwards, move the pipette as close as possible and before touching the cell, shift to the higher magnification objective, such as 40x or greater; 6) Some groups use a pneumatic microinjector. Pneumatic injection is not the best choice since it is not precise and could inject unknown volumes; with a pulse the protocol could be standardized to inject a known volume of dye. Additionally, it is recommended to inject into the membrane above the cytosol because of the depth; injection in the membrane above the nucleus could injure it and affect cell physiology. Finally, cells that are not too flat are preferable over flat ones.
In summary, this method is effective to study intercellular communication by gap junctions but needs expertise/experience and good material and equipment to obtain high quality data. We hope that this article and video help beginners to understand and perform this technique.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors dedicate this paper in honor of Prof. Gilberto Oliveira-Castro who introduced research in intercellular communication by gap junctions in Brazil. This work was funded by Capes, CNPQ and Faperj.
Materials
| Lucifer yellow | Sigma | L0259 | |
| Lithium Chloride | Sigma | L4408 | |
| PBS tablets | Sigma | P4417 | |
| RPMI | Sigma | R4130 | |
| Bovine fetal serum | Cultilab | ||
| Trypsin | Sigma | T4799 | |
| Microscope | Nikon | TE-2000 | For microinjection experiments, one needs an inverted fluorescence microscope and filters for fluorescent microscopy |
| vibration-insulated table | Newport | VH3036W-OPT | A vibration-insulated table is needed to protect the experiments from vibration and avoid cell damage |
| Micromanipulator | Narishige | MMO-203 | This equipment allows precision adjustments of the micropipette, which is needed for cell micro injection. |
| Current Generator | Digitimer | DS2 | To produce the dye flow through the micropipette, a current below one nano ampere was given using a current generator with an electrode inside the micropipette or an amplifier which has a capacitance compensation circuit (old electrometer) or current injection functions of new patch clamp amplifiers, and the ground wire submersed in the plate dish. Alternatively, the dye can be injected by a pneumatic microinjector, following the factory recommendations. |
References
- Bennett, M. V., et al. Gap junctions: new tools, new answers, new questions. Neuron. 6 (3), 305-320 (1991).
- Orellana, J. A., Martinez, A. D., Retamal, M. A. Gap junction channels and hemichannels in the CNS: Regulation by signaling molecules. Neuropharmacology. , (2013).
- Peracchia, C. Structural correlates of gap junction permeation. Int Rev Cytol. 66, 81-146 (1980).
- Loewenstein, W. R. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 560 (1), 1-65 (1979).
- Alves, L. A., et al. Functional gap junctions in thymic epithelial cells are formed by connexin 43. Eur.J Immunol. 25 (2), 431-437 (1995).
- Alves, L. A., et al. Are there functional gap junctions or junctional hemichannels in macrophages?. Blood. 88 (1), 328-334 (1996).
- Fonseca, P. C., et al. Characterization of connexin 30.3 and 43 in thymocytes. Immunology letters. 94 (1-2), 65-75 (2004).
- Nihei, O. K., et al. Modulatory effects of cAMP and PKC activation on gap junctional intercellular communication among thymic epithelial cells. BMC Cell Biol. 11, 3 (2010).
- Czyz, J., Szpak, K., Madeja, Z. The role of connexins in prostate cancer promotion and progression. Nat Rev Urol. 9 (5), 274-282 (2012).
- El-Saghir, J. A., El-Habre, E. T., El-Sabban, M. E., Talhouk, R. S. Connexins: a junctional crossroad to breast cancer. Int J Dev Biol. 55 (7-9), 773-780 (2011).
- Cea, L. A., et al. Connexin- and pannexin-based channels in normal skeletal muscles and their possible role in muscle atrophy. J Membr Biol. 245 (8), 423-436 (2012).
- Cotrina, M. L., Nedergaard, M. Brain connexins in demyelinating diseases: therapeutic potential of glial targets. Brain Res. 1487, 61-68 (2012).
- Park, H. a. n. -. A., R, S., Khanna, S. a. v. i. t. a., Sen, C. h. a. n. d. a. n. . K. Current Technologies in Single-Cell Microinjection and Application to Study Signal Transduction. Methods in Redox Signaling. , (2010).
- Abbaci, M., Barberi-Heyob, M., Blondel, W., Guillemin, F., Didelon, J. Advantages and limitations of commonly used methods to assay the molecular permeability of gap junctional intercellular communication. Biotechniques. 45 (1), 33-52 (2008).
- Stewart, W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 14 (3), 741-759 (1978).
- Meda, P. Probing the function of connexin channels in primary tissues. Methods. 20 (2), 232-244 (2000).
- Klaunig, J. E., Shi, Y. Assessment of gap junctional intercellular communication. Curr Protoc Toxicol. , (2009).
- Hanani, M. Lucifer yellow – an angel rather than the devil. J Cell Mol Med. 16 (1), 22-31 (2012).
- Orci, L., Biochemistry, C. Blockage of Cell-to-Cell Communication within Pancreatic Acini Is Associated with Increased Basal Release of Amylase Materials and Methods Preparation of Acini. Cell. 103 (August), 475-483 (1986).
- Hitomi, M., et al. Differential connexin function enhances self-renewal in glioblastoma. Cell Rep. 11 (7), 1031-1042 (2015).
- Nihei, O. K., Campos de Carvalho, ., C, A., Spray, D. C., Savino, W., Alves, L. A. A novel form of cellular communication among thymic epithelial cells: intercellular calcium wave propagation. Am J Physiol Cell Physiol. 285 (5), C1304-C1313 (2003).
- Kanno, Y., Loewenstein, W. R. Intercellular Diffusion. Science. 143 (3609), 959-960 (1964).
- Alves, L. A., Nihei, O. K., Fonseca, P. C., Carvalho, A. C., Savino, W. Gap junction modulation by extracellular signaling molecules: the thymus model. Braz J Med Biol Res. 33 (4), 457-465 (2000).

