An Anoxia-starvation Model for Ischemia/Reperfusion in C. elegans
Summary
A protocol is described that uses anoxia/starvation in C. elegans to model ischemia/reperfusion. Functional outcomes include increased mortality, visible abnormalities in GFP-labeled neuronal processes, and impaired behavioral responses that require neuronal function.
Abstract
Protocols for anoxia/starvation in the genetic model organism C. elegans simulate ischemia/reperfusion. Worms are separated from bacterial food and placed under anoxia for 20 hr (simulated ischemia), and subsequently moved to a normal atmosphere with food (simulated reperfusion). This experimental paradigm results in increased death and neuronal damage, and techniques are presented to assess organism viability, alterations to the morphology of touch neuron processes, as well as touch sensitivity, which represents the behavioral output of neuronal function. Finally, a method for constructing hypoxic incubators using common kitchen storage containers is described. The addition of a mass flow control unit allows for alterations to be made to the gas mixture in the custom incubators, and a circulating water bath allows for both temperature control and makes it easy to identify leaks. This method provides a low cost alternative to commercially available units.
Introduction
C. elegans is a nematode that has been widely adopted as a multicellular eukaryotic model organism since its introduction by Brenner1. It is a cheap, simple, and versatile model, which allows easy links between genetic alterations and phenotypic changes2.
Ischemia is characterized by a lack of nutrients and oxygen supply to a tissue, followed by reperfusion, when a burst of reactive oxygen species is produced3 and most of the damage occurs. In 2002, a model of ischemia/reperfusion (IR) in C. elegans was developed4 involving submitting the whole worm to anoxia, nutrient deprivation and heat stress for approximately 20 hr followed by 24 hr under normal conditions. Although this model is technically an anoxia-starvation (AS) condition, cell death occurs through mechanisms that are conserved in mammals, including damage induced by oxidants during reperfusion5. Furthermore, similar to mammalian IR, damage induced by AS in C. elegans can be prevented by ischemic preconditioning6,7 or anesthetic preconditioning8,9.
The protocols below demonstrate how to mimic IR in C. elegans using the AS model, how to score morphological and behavioral abnormalities that result from AS, and how to adapt the protocol in a way that allows the experiment to be conducted with a lower initial investment using a custom-made, easily-constructed chamber alternative.
Protocol
1. C. elegans Growth
- Prepare 35 mm Nematode Growth Media (NGM) agar plates seeded with OP50 bacteria, as per standard cultivation methods1.
- Synchronize C. elegans by placing 6 gravid adults onto a seeded NGM plate. Remove the adults after ~100 eggs have been laid (~3 hr).
- Incubate the plates for 3 days at 20 °C, at which point the worms will have developed to young adult stage and are optimal for this experiment. Alternative protocols to synchronize C. elegans are described elsewhere10.
2. Materials for AS
- M9 buffer (22 mM KH2PO4, 42 mM Na2HPO4, 86 mM NaCl, 1 mM MgSO4) should be purged of oxygen by equilibrating with N2 (argon can also be used) during 30 min prior to the experiment and kept surrounded by ice.
- Make a small hole (3 mm wide) in the lid of 1.5 ml microcentrifuge tubes in which the worms will be placed during AS. The hole allows for gas exchange without keeping the lid open, as this can lead to excessive media evaporation.
3. Anoxia/Starvation
- Conduct all experiments at least in triplicate.
- Add 1 ml of RT, oxygenated M9 buffer to each 35 mm plate containing young adult worms grown as described above. Be careful to avoid removing bacteria from the plate (add M9 to the edges where bacteria weren't seeded). After ~1 min the worms should be swimming in the M9.
- Coat a 1 ml pipette tip with BSA by pipetting and expelling a sterile 1% BSA solution.
- Carefully incline the plate containing the worms in M9 buffer, remove the M9 from the same place where it was added, and place the suspension in the prepared microcentrifuge tubes. Let rest in ice until the worms have dropped to the bottom of the tubes (~1-2 min).
- Remove the maximum amount of M9 possible without disturbing the worms from the tube (leaving around 100 μl), add 1 ml of the oxygen-purged and iced M9 and place the tube over ice until the worms settle to the bottom again. Repeat the last step 3x. In the last wash, remove the M9 until ~100 μl remains in the tube.
- Place the tubes with the worms in deoxygenated M9 inside an anoxic/hypoxic chamber (see instructions below, Section 3.6.1) or, alternatively, into a custom sealed container (see instructions below, Section 3.6.2).
- If a commercial anoxic/hypoxic chamber is to be used, leave some purged M9 inside the chamber before starting step 3.5 and repeat step 3.5 once the worms are inside the chamber.
- If a custom chamber is to be used, a detailed description of how to create a low-cost apparatus is presented at the end of this protocol and has been described by another group previously11. Briefly:
- Make two holes in a 250 ml Tupperware-type container lid and add tube connectors to them. One will be used to inject the gas mixture while the other will be the gas exit into a water bath.
- Place the worms into the chamber and seal the chamber using the lid.
- Gas with a constant N2 flux (100 ml/min).
- Place the container inside a water bath at 26 °C. Ensure that the exit tube is well immersed in water so no air returns and that there are no leaks in the system (apparent by bubbles arising from the chamber itself).
- Incubate the worms at 26 °C for 20 hr.
4. Simulated Reperfusion
- After 20 hr under AS conditions, remove the tubes from the incubation chamber into room air.
- Pipette C. elegans with a BSA-coated tip (as in step 3.3) onto an OP50 seeded 35 mm NGM plate. Incubate the plates at 20 °C for 24 hr. After this, the worms will be ready to be scored.
5. Identifying Dead Versus Live Worms
- Using a platinum pick, lightly touch the top of the head of the worm. Live worms will move backwards after being touched. If the worms are nonresponsive, score as dead. Occasionally, a worm will not move backwards, but may exhibit a slight side-to-side head movement. Although not technically dead, these worms are almost certain to expire within hours, and should be scored as dead. If one waits too long to score the worms, internal hatching of progeny can occur and these “bags-of-worms” can rupture, making detecting the carcasses very difficult.
- Remove both live and dead worms from the plate as they are counted to avoid duplicate counts. The live worms can be moved to a separate plate to perform behavioral assays. The amount of living worms relative to the total is represented as the % of surviving worms (Figure 1A).
6. Touch Response Assay
- A detailed description of this protocol has been presented in Hart, Anne C., ed. Behavior (July 3, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.87.1, http://www.wormbook.org.
- To score for touch response, identify worms that are moving and lightly touch the side of the worm's head (near the middle part of the pharynx) with an eyelash pick (Figure 3).
- If the worm moves backward, score as responsive, if not, score as nonresponsive (Figure 1B). Repeat this step 10-15x for each worm with 10 sec intervals. Probe at least 10 worms in each group to have accurate data. The same assay can be used to assess forward locomotory changes following light touch to the posterior body wall. Be sure not to touch the head or tail of the worm, since this stimulates a distinct behavior.
- Finally, incorporate genetic control strains [N2 Bristol wild-type for positive, mec (mechanosensory) mutant for negative - e.g. CB1338 mec-3 (e1338)IV] to calibrate the force with which to touch the worms. Too harsh of a stimulation will elicit a behavioral response in mec mutants, too little will not elicit an effect in wildtype N2 controls.
7. Neuronal Modifications
- To visualize post AS neuronal modifications, use a strain which expresses GFP in the touch-responding mec neurons4,5. These neurons have long processes that run along the body wall and are easily scored for morphological abnormalities. In addition, the morphologic information can be integrated with behavioral measures of neuronal function, as described above. One strain that can be used for this purpose is TU2583, which is available from the C. elegans Genetics Center and contains the uIs25 integrated transgene expressing GFP from the mec-18 promoter, or alternatively TU2562, which contains an integrated mec-3::GFP fusion.
- Prepare an agarose pad.
- Melt 2% agarose in water, bring to 1x M9 with a 10x stock, and maintain the solution at 70 °C.
- Place a drop (~20 μl) of this solution on a glass slide (25 mm x 75 mm) that has been placed between two other slides, each of which have a single piece of tape on their bottom side.
- Place a final glass slide perpendicular to the first on top of the 2% agarose solution, and let it cool at RT. This should form a pad that is the thickness of the tape.
- Remove the top slide and add 10 μl of M9 containing 0.1% tetramisole, which will immobilize the worms.
- Move some (~10) live worms onto the 2% agarose slide. Place a coverslip over them and visualize GFP using a fluorescence microscope under a 100X objective with the appropriate illumination.
- Damaged neurons will show a cytosolic membrane string of pearls pattern in the processes, comprised of multiple punctum (Figure 2A), and/or abnormalities where the processes appear to be broken (Figure 2B). Count punctum and abnormalities in both neurons for each worm, using a total of at least 10 worms.
8. Lab-made Hypoxic Chamber
- A Tupperware-style, sealable, airtight container can be retrofit as an anoxic chamber. Take care to select a container that is as small as feasible. A small container creates a hypoxic environment faster and is easier to fit in the water bath (Figure 4).
- Make two holes on the lid (can be done with a screwdriver or by heating forceps and forcing into the lid) and insert a plastic or metal connector that fits the tubing (Figure 5A). Use glue that can be placed under water, and also gets hard after drying, avoiding movement of the connector and hence reducing the possibility of leakage (Figure 5B).
- The container should be placed into the water bath so that it is submerged completely (Figure 4). To immerse the container, use a bottle weight or a diving weight.
- One of the tube connections will be used to introduce a gas mixture and the other tube will act as a pressure relief, hence it should be left under the water so that the container is sealed off from the external environment (Figure 4).
- The air composition can be modulated by using custom made gas, mass flow controllers or any apparatus that mixes the desired gases. The gas flow should be controlled, as high flow can create excessive evaporation. For example, in a 250 ml container, 100 ml/min of gas will provide good space for the worms and a suitable flux.
- The length of the tubing should be kept to a minimum, as they can be permeable to gas, allowing the entrance of O2. It is important to avoid using silicone, Tygon tubes as these materials are permeable to gas exchange. We prefer polypropylene or nylon tubes. Glass or metal tubing maintain the gasses well, but are also harder to work with.
Representative Results
Subjecting C. elegans to 20 hr of AS at 26 °C in a custom lab-made incubation chamber as described (Section 3.6.2) resulted in significant mortality (Figure 1A)5. Subsequent fluorescent imaging of punctum and breaks in the GFP labeled neuronal processes of survivors confirmed the presence of morphological abnormalities (Figure 2). The survivors also responded poorly to light body wall touch (Figure 1B). This model has been used by multiple groups to study how genetic predisposition, pharmaceutical intervention, and metabolic plasticity affects AS dependent outcomes4-6,8,9,12,13.
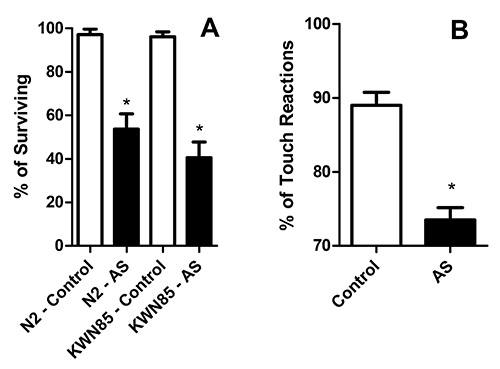
Figure 1. C. elegans survival and touch response after AS. A) Percentage of worms that exhibit 24 hr post-AS survival. The N2 strain is the wild type genetic background, and KWN85 contains an integrated transgene that labels mec neurons with GFP. B) Response to touch stimuli of living C. elegans (N2 Strain) after AS. Please click here to view a larger version of this figure.
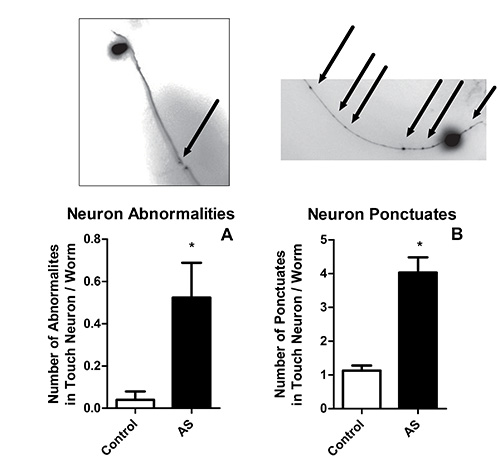
Figure 2. Touch neuron modifications after AS. Touch neuron (PLML and PLMR) abnormalities such as tortuous processes and breaks (A) or the accumulation of GFP aggregates in the processes (B) were monitored in surviving anesthetized C. elegans after AS.
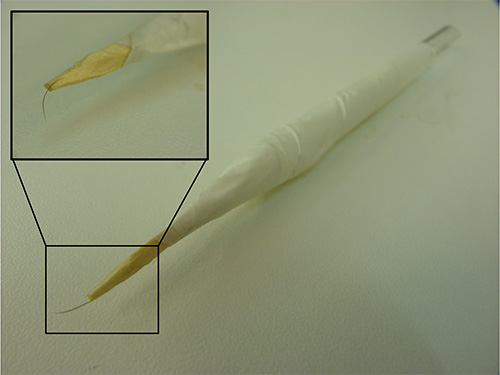
Figure 3. Eyelash pick. An assembled eyelash pick. In the inset, a detail of the eyelash glued to the toothpick wood.
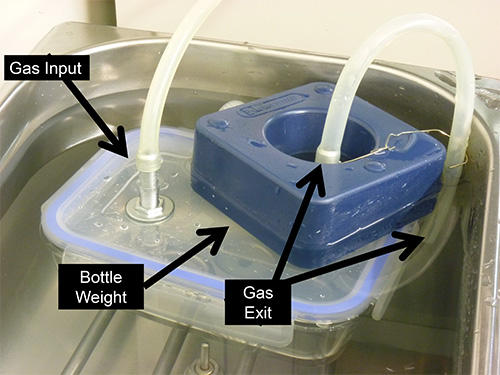
Figure 4. Lab-made hypoxic chamber inside the water bath. A closed container ready to start the experiment. Arrows indicate the gas entrance and exit and the bottle weight used to keep it from floating.
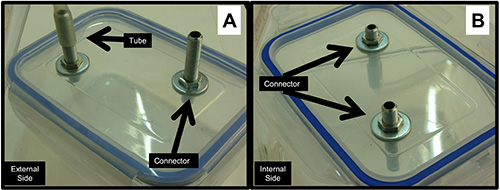
Figure 5. Details of the internal and external side of the container lid. A) External side of the container lid, indicating the tube and its connector attached to the previously made hole. B) External side of the container lid, indicating the tube connector attached to the previously made hole.
Discussion
AS has been widely used in C. elegans to model IR injury. Some key points should be highlighted for this protocol: C. elegans are resistant to a wide array of injuries, justifying the need for 3 concomitant insults (heat, starvation and anoxia) to achieve death using this system. Anoxia alone does not kill the worms in this window of time14. Furthermore, temperature increase is an additional stress, so it is important to monitor closely. Strictly speaking, starvation does not contribute significantly to the degree of mortality observed7, per se, but it appears to reduce variability among experimental replicates. Given that there can be significant variability from day-to-day, it is extremely important to compare samples run directly in parallel, and to repeat experiments over multiple days. In general, outcomes are measured for three separate plates of 50-100 worms/experimental condition and these are then averaged and considered as a single experimental replicate. Generally, between seven and nine replicates appear to be sufficient to achieve or rule out statistical significance.
The developmental stage of the worms used in the experiment also needs to be carefully monitored as the susceptibility of different stages to AS damage varies significantly15,16. The use of young adults is standard, and larval stage (L3 and L4) worms appear to be more resistant to the damaging effects of AS (unpublished data).
This protocol presents two ways to perform the experiments, one using a lab-made apparatus (using Tupperware-type containers and gas input11) and other using a commercial hypoxic chamber4,6. Anoxia can be achieved by other means that consume the oxygen, as described elsewhere13,17. The use of alternate techniques to create a hypoxic environment may change the AS incubation time necessary to create the desired amount of death. Targeting ~20% survival is an ideal starting point for studying protective interventions, while 80% is similarly ideal for interventions that exacerbate the detrimental effects of AS. Another important caveat is the time at which the observer scores dead/alive worms. If the time for analysis is extended beyond 24 hr, the data may be misleading since dead worms become increasingly difficult to identify. This may be due to worm carcasses becoming relatively transparent over time, but also to the fact that fertilized embryos can develop into progeny post-mortem inside of the carcasses and disrupt them as they emerge.
The analysis of neuronal morphology can be modified to look at protein expression patterns18, nuclear fragmentation4 and other parameters19 by substituting a worm strain that expresses the appropriate genetically encoded marker. One final caveat is that the visualization of the neuronal processes should be done less than 30 min after placing the worms on the slide. Animals kept under anesthesia on slides for longer periods can exhibit AS-independent damage. Adjust the amount of animals per slide according to the time needed to track and analyze them.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Figures 1 and 2 were previously published in Free Radical Biology & Medicine (Queliconi et al.5) and have copyright held by Elsevier. This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), the Instituto Nacional de Ciência e Tecnologia de Processos Redox em Biomedicina, the Núcleo de Apoio à Pesquisa de Processos Redox em Biomedicina, USPHS NS064945 (K.N.), and USPHS GM087483 (K.N.). B.B.Q. is a doctoral student supported by a FAPESP fellowship.
Materials
| N2 strain | CGC (http://www.cbs.umn.edu/CGC/) | Wild type strain | |
| TU2583 uIs25 (Pmec-18::GFP) | CGC (http://www.cbs.umn.edu/CGC/) | TU2583 | integrated fluorescent transgene used to label touch neurons |
| CB1338 mec-3 (e1338)IV | CGC (http://www.cbs.umn.edu/CGC/) | CB1338 | canonical mec-3 mutant that is touch insensitive |
| Microcentrifuge Tube | Eppendorf | 0030 120.086 | |
| Nikon Eclipse TE2000-U Microscope | Nikon USA | TE2000-U | |
| Low Temperature Incubator | Sheldon Manufacturing Inc. | Model 2005 | |
| Eyelash Pick | An eyelash pick can be prepared by attaching an eyelash onto a wooden toothpick, then attaching the toothpick in a glass Pasteur pipette (Figure 3) | ||
| Hypoxic Chamber | Coy | 8307030 | Hypoxic Glove box equipped with paladium catalyst and CO2 controller. |
References
- Brenner, S. The genetics of Caenorhabditis elegans. 유전학. 77 (1), 71-94 (1974).
- Maine, E. M. Studying gene function in Caenorhabditis elegans using RNA-mediated interference. Briefings Funct. Genom. Proteom. 7 (3), 184-194 (2008).
- Vanden Hoek, ., L, T., Shao, Z., Li, C., Zak, R., Schumacker, P. T., Becker, L. B. Reperfusion injury on cardiac myocytes after simulated ischemia. Am. J. Physiol. 270 (4), 1334-1341 (1996).
- Ba Scott, ., Avidan, M. S., Crowder, C. M. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science. 296 (5577), 2388-2391 (2002).
- Queliconi, B. B., Marazzi, T. B. M., et al. Bicarbonate modulates oxidative and functional damage in ischemia-reperfusion. Free Rad. Biol. Med. 55, 46-53 (2013).
- Wojtovich, A. P., DiStefano, P., Sherman, T., Brookes, P. S., Nehrke, K. Mitochondrial ATP-sensitive potassium channel activity and hypoxic preconditioning are independent of an inwardly rectifying potassium channel subunit in Caenorhabditis elegans. FEBS Lett. 586 (4), 428-434 (2012).
- Dasgupta, N., Patel, A. M., Scott, B. A., Crowder, C. M. Hypoxic Preconditioning Requires the Apoptosis Protein CED-4 in C. elegans. Curr. Biol. 17 (22), 1954-1959 (2007).
- Jia, B., Crowder, C. M. Volatile anesthetic preconditioning present in the invertebrate Caenorhabditis elegans. Anesthesiology. 108 (3), 426-433 (2008).
- Wojtovich, A. P., Sherman, T. A., Nadtochiy, S. M., Urciuoli, W. R., Brookes, P. S., Nehrke, K. SLO-2 is cytoprotective and contributes to mitochondrial potassium transport. PloS One. 6 (12), e28287 (2011).
- Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A., Cerón, J. Basic Caenorhabditis elegans methods: synchronization and observation. J. Vis. Exp. (64), e4019 (2012).
- Fawcett, E. M., Horsman, J. W., Miller, D. L. Creating defined gaseous environments to study the effects of hypoxia on C. elegans. J. Vis. Exp. (65), e4088 (2012).
- Butler, J. A., Mishur, R. J., Bokov, A. F., Hakala, K. W., Weintraub, S. T., Rea, S. L. Profiling the anaerobic response of C. elegans using GC-MS. PLoS One. 7 (9), 10-1371 (2012).
- Padilla, P. A., Nystul, T. G., Zager, R. A., Johnson, A. C., Roth, M. B. Dephosphorylation of cell cycle-regulated proteins correlates with anoxia-induced suspended animation in Caenorhabditis elegans. Mol. Biol. Cell. 13 (5), 1473-1483 (2002).
- Jiang, H., Guo, R., Powell-Coffman, J. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc. Natl. Acad. Sci. U.S.A. 98 (14), 7916-7921 (2001).
- Twumasi-Boateng, K., Wang, T. W., et al. An age-dependent reversal in the protective capacities of JNK signaling shortens Caenorhabditis elegans lifespan. Aging Cell. 11 (4), 659-667 (2012).
- Wang, Y., Chen, J., Wei, G., He, H., Zhu, X., Xiao, T., Yuan, J., Dong, B., He, S. S. k. o. g. e. r. b. &. #. 2. 4. 8. ;. G., Chen, R. The Caenorhabditis elegans intermediate-size transcriptome shows high degree of stage-specific expression. Nucleic Acids Res. 39 (12), 5203-5214 (2011).
- Perry, C. N., Huang, C., Liu, W., Magee, N., Carreira, R. S., Gottlieb, R. Xenotransplantation of mitochondrial electron transfer enzyme, Ndi1, in myocardial reperfusion injury. PloS One. 6 (2), e16288 (2011).
- Chai, Y., Li, W., Feng, G., Yang, Y., Wang, X., Ou, G. Live Imaging of Cellular Dynamics During Caenorhabditis elegans Postembryonic Development. Nature Protoc. 7 (12), 2090-2102 (2012).
- Nehrke, K. A reduction in intestinal cell pHi due to loss of the Caenorhabditis elegans Na+/H+ exchanger NHX-2 increases life span. J. Biol. Chem. 278 (45), 44657-44666 (1074).

