Using Fluorescent Proteins to Monitor Glycosome Dynamics in the African Trypanosome
Summary
Glycosome dynamics in African trypanosomes are difficult to study by traditional cell biology techniques such as electron and fluorescence microscopy. As a means of observing dynamic organelle behavior, a fluorescent-organelle reporter system has been used in conjunction with flow cytometry to monitor real-time glycosome dynamics in live parasites.
Abstract
Trypanosoma brucei is a kinetoplastid parasite that causes human African trypanosomiasis (HAT), or sleeping sickness, and a wasting disease, nagana, in cattle1. The parasite alternates between the bloodstream of the mammalian host and the tsetse fly vector. The composition of many cellular organelles changes in response to these different extracellular conditions2-5.
Glycosomes are highly specialized peroxisomes in which many of the enzymes involved in glycolysis are compartmentalized. Glycosome composition changes in a developmental and environmentally regulated manner4-11. Currently, the most common techniques used to study glycosome dynamics are electron and fluorescence microscopy; techniques that are expensive, time and labor intensive, and not easily adapted to high throughput analyses.
To overcome these limitations, a fluorescent-glycosome reporter system in which enhanced yellow fluorescent protein (eYFP) is fused to a peroxisome targeting sequence (PTS2), which directs the fusion protein to glycosomes12, has been established. Upon import of the PTS2eYFP fusion protein, glycosomes become fluorescent. Organelle degradation and recycling results in the loss of fluorescence that can be measured by flow cytometry. Large numbers of cells (5,000 cells/sec) can be analyzed in real-time without extensive sample preparation such as fixation and mounting. This method offers a rapid way of detecting changes in organelle composition in response to fluctuating environmental conditions.
Introduction
Trypanosoma brucei causes African sleeping sickness in humans and a wasting disease, nagana, in cattle. Drugs used in the treatment of these diseases are antiquated and extremely toxic, vaccines are not available, and the potential for the development of drug resistance necessitates the search for new drug targets1.
During its lifecycle, T. brucei, alternates between an insect vector and mammalian host; two hosts that present very different environments in which the parasite must survive. A number of metabolic and morphological changes occur as the parasite is exposed to different environmental conditions. Some of the most dramatic changes are observed in single-membrane-bounded parasite specific microbodies, termed glycosomes13.
Glucose levels are relatively high (~5 mM) in the bloodstream and bloodstream parasites (BSF) generate ATP exclusively through glycolysis while mitochondrial metabolism is repressed14. Unlike other eukaryotes in which glycolysis occurs in the cytoplasm, T. brucei compartmentalizes most of the glycolytic enzymes in glycosomes14,15. The parasites are taken up by the tsetse fly during a bloodmeal and experience a drop in glucose, which falls to undetectable levels within 15 min of being ingested by the fly. The metabolism of insect, procyclic form (PCF), parasites is more flexible and glucose, as well as amino acids such as proline, can be used in the synthesis of ATP16-18. Comparative proteomic studies reveal lifecycle dependent changes in glycosomal and mitochondrial proteins with glycolytic proteins increased in bloodstream parasites and mitochondrial proteins involved in TCA cycle and respiratory chain13,19. While many studies have focused on the differences between BSF and PCF glycosomes, little is known about the changes in PCF glycosomes that occur in response to environmental changes.
In the hindgut of the fly, glucose levels are low with transient increases during a feeding20. In most in vitro studies, PCF parasites are grown in media containing glucose. However, recent studies have demonstrated that PCF metabolism changes significantly in response to glucose availability17. In the absence of glucose, proline uptake and proline dehydrogenase activity increase18. This change in mitochondrial metabolism is likely accompanied by a change in glycosome composition and morphology, however, this has not been directly assessed.
Electron and fluorescence microscopy are common techniques used to study glycosome dynamics in T. brucei2,21-24. These protocols are time and labor intensive, expensive, and difficult to adapt to real-time studies and high throughput protocols. To overcome this limitation, a fluorescent-organelle reporter system used to study organelles in mammalian and yeast systems has been modified for use in T. brucei12.
Fluorescent-organelle reporter systems have been extensively used in higher eukaryotes such as yeast, plant, and mammalian cells25-27. In such systems, a fluorescent protein is fused to an amino acid sequence that targets the protein to specific organelles. The degradation or synthesis of the targeted proteins is measured via fluorescence and changes in organelle composition are reflected by changes in cell fluorescence.
When the open reading frame of enhanced yellow fluorescent protein (eYFP) is fused to a type II peroxisomal targeting sequence (PTS2)12, the PTS2eYFP protein is imported into mature, import-competent glycosomes and fluorescence can be monitored via flow cytometry. Variations in glycosome composition are reflected by changes in cellular fluorescence. This system can aid in resolving the mechanisms that regulate environmentally induced changes in glycosome composition.
This manuscript describes the generation of a glycosome reporter system in PCF parasites in conjunction with flow cytometry to monitor real-time glycosome dynamics in live parasites and provides an example of how it has been used to follow changes in glycosome composition in response to different environments. In summary, glycosome composition is influenced by extracellular glucose concentrations and passage of log-phase cultures into fresh media triggers changes in glycosome composition. This system can be modified to study the dynamic behavior of other organelles in trypanosomes and other parasites.
Protocol
1. General Trypanosome Husbandry
- Weigh solids for SDM79 media preparation (Table 1).
- Store at 4 °C in 50 ml conical or a Ziploc bag. NOTE: Reagents are stable for at least 6 months.
- Thaw fetal bovine serum (FBS) in a 37 °C waterbath, and mix periodically by inversion. NOTE: FBS is received from the supplier as a sterile solution. Filter sterilizing FBS reduces its ability to support parasite growth.
- Once the entire bottle is thawed, heat inactivate serum for 30 min in a 56 °C waterbath, mixing periodically to minimize precipitation of serum components.
- Thaw penicillin/streptomycin solution (pen/strep), hemin (2 mg/ml in 50 mM NaOH), and basal medium eagle vitamin solution at room temperature.
- To a 1,000 ml graduated cylinder, add 20.2 g of SDM79 solids (Table 1) to the liquid components (Table 2) and mix well on a stir plate.
- Adjust pH to 7.35, and bring volume to 850 ml with water.
- Filter sterilize media by attaching a vacuum hose to the bottom of a filter bottle. Apply vacuum until the solution is filtered.
- Remove filter top in the biosafety cabinet and add 150 ml of heat inactivated FBS. Remove plastic covering from sterile cap and seal media bottle.
- Prepare SDM80 media in the same way as SDM79 with the following exceptions; do not add glucose or glucosamine and use MEM without glutamine. In place of 150 ml of heat inactivated FBS, add 135 ml of dialyzed, heat inactivated FBS, and 15 ml of heat inactivated FBS.
- Culture PCF parasites in 25 cm2 flask with 10 ml appropriate medium at 27 °C and 5% CO2. NOTE: Cells should be counted daily using a hemocytometer or a flow cytometer (see section 6) and cultures should be maintained at densities between 1 x 105 and 5 x 106 cells/ml.
2. Transfection of PCF Parasites with the Fluorescent Reporter Construct
NOTE: To follow glycosome dynamics, a reporter protein containing the peroxisomal targeting sequence (PTS2) of aldolase fused to enhanced yellow fluorescent protein is expressed in the parasites. The sequence encoding the fusion protein is cloned into pXS2bla12, which contains the procyclin promoter and the tubulin intergenic regions, which direct homologous recombination into the genome and the blasticidin resistance gene for selection. Procyclic cell lines harboring the genes encoding the T7 polymerase and tetracycline repressor (PF29-13) are transformed with the targeting construct, pXS2:PTS2eYFP.
- Prepare cytomix by mixing the components listed in Table 3. Filter sterilize and store at RT.
- Linearize plasmid DNA with MluI by pipetting 10 μl of DNA (1 μg/μl), 5 μl restriction enzyme buffer, 33 μl water, and 2 μl of MluI enzyme into a microcentrifuge tube. Incubate at 37 °C for 1 hr.
- Purify the restriction digest by adding 1 volume of binding buffer to the restriction enzyme digest. Mix binding buffer and digested DNA by vortexing. Briefly centrifuge to collect the sample at the bottom of the tube.
- Insert a DNA binding column into a 2 ml collection tube, and transfer digested DNA/binding buffer solution to column.
- Centrifuge for 10,000 x g for 1 min. After centrifugation, discard filtrate from collection tube and return the column to the collection tube.
- Add 700 µl of SPW wash buffer and centrifuge at 10,000 x g for 1 min.
- Discard filtrate from collection tube, return the column to the collection tube and repeat SPW wash step and centrifugation.
- Discard filtrate, return column to collection tube, and centrifuge empty column for 3 min at 10,000 x g to remove residual ethanol.
- In a biosafety cabinet, throw away collection tube and place column in a sterile microcentrifuge tube.
- To elute the DNA, add 25 µl of sterile water to column and incubate at room temperature for 2 min. Centrifuge at 10,000 x g for 1 min.
- Add another 25 µl of sterile water in biosafety hood to column and incubate at room temperature for 2 min.
- Centrifuge at 10,000 x g speed for 1 min.
- In a biosafety cabinet transfer entire volume of DNA to a new sterile microcentrifuge tube. NOTE: This step prevents contamination from the open lid during centrifugation.
- Add the sterile, purified, linearized DNA (10 µg) to 400 µl of filter-sterilized cytomix.
- Count cells as described in section 6 and harvest 5 x107-108 cells by centrifugation at 800 x g for 15 min at RT.
- Pour off supernatant and resuspend the cell pellet in 450 µl of DNA + cytomix using a 1 ml serological pipette.
- Transfer solution containing cells, DNA, and cytomix to a sterile 4 mm gap cuvette and place cuvette in the electroporation chamber.
- Select “Exponential decay” and manually enter the following settings: Voltage: 1.5 kV, Capacitance: 25 mF, Resistance: Ω, and Cuvette: 4 mm. Press pulse. Once the pulse is complete, remove cuvette from the electroporation chamber and return to the biosafety cabinet.
- Into a new sterile flask, pipette 10 ml of SDM79. Transfer the transformed cells to the flask with 10 ml of SDM79.
- Incubate WITHOUT drug selection for 24 hr at 27 °C, 5% CO2. After 24 hr, add G418 (15 µg/ml), hygromycin (50 µg/ml), and blasticidin (10 µg/ml). Pass 1 ml of transformed cells with drug into 9 ml of SDM79 with drug.
- Analyze cells daily as described in sections 6 and 7. When cells are fluorescent, and have reached a cell density of 1 x 107/ml, make stabilates for long-term storage (3.1-3.3).
3. Making Stabilates
- To make freezing media, add an equal volume of 100% glycerol to cytomix and filter sterilize.
- Add 200 µl of freezing media to 800 µl of cells (1 x 107) in a cryovial, mix gently by inversion and place between two Styrofoam racks and freeze at -80 °C.
- Once frozen (~24 hr), transfer cells to liquid nitrogen. NOTE: It is important to move cells into LN2 after 24 hr, as longer storage at -80 °C leads to a decrease in cell viability.
4. Thawing Frozen Stocks
- Remove frozen vial from -80 °C and thaw at RT for approximately 10 min.
- Pipette 9 ml of SDM79 with drug into a new sterile flask. Add thawed cells to this flask. Pass cells 1:10 by adding 1 ml of this culture to 9 ml of SDM79 in a new flask (final concentration of 1 x 105/ml).
- Before the cells reach a density of 107/ml, begin cytometer setup and dilution assays.
5. Cytometer Setup
- Turn on the flow cytometer and open CFlow Plus program. NOTE: Before running any samples, the fluidics should be backflushed and rinsed according to steps 5.2-5.7.
- Replace the tube of nanopure water in which the sip is stored with an empty tube.
- Select the “backflush” button.
- Once backflush is complete, remove microcentrifuge tube from sip and discard.
- Place a new microcentrifuge tube containing 1 ml of new nanopure water on the sip.
- Select a new well in CFlow program. Under “Run Limits” set time for 2 min. Under “Fluidics” select “fast” and “Run”.
- Once the 2 min time limit is complete, click the “Delete Sample Data” button.
6. Cell Counting using the Flow Cytometer
- Replace water with the sample to be counted. Set “Run Limits” to 30 sec, “Fluidics” to “fast” and then select “Run”.
- After the 30 sec time limit is complete, CFlow plus will provide the events/μl under “Last Run.” Repeat count for each culture. NOTE: Before cells reach 1 x 107 cells/ml, proceed with dilution assay. Cells should be discontinued after one dilution assay is completed. Cells cultured for longer periods lose their ability to respond to environmental conditions.
7. Measuring Fluorescence using the Flow Cytometer
- To assess fluorescence, set “Run Limits” to 10,000 events, and “Fluidics” to “slow”. Select “Set threshold”. Under “Primary Threshold”, select “Permanently eliminate events on”, “FSC-H” (in drop down box) and enter less than 30,000. Click “apply”, then “close”.
- Select the “Histogram” button in one of the new plot windows and select “FSC-A” From the drop down list, select “FL1-A”. NOTE: FL1-A measures fluorescence in the 530 nm wavelength.
- Select “Run” and repeat for all cultures.
- Run cleaning solution through the fluidics line for 2 min and water for 2 min.
8. Dilution Assays
- Pass cells to a density of 1 x 105 cells/ml in 3 ml of SDM79 in a 25 cm2 culture flask and measure florescence immediately after passage and then at 3 hr and 24 hr.
9. Data Analysis
- Select “Analyze” tab on CFlow Plus.
- Click “Histogram” button under “Make a new plot.” Select “FSC-A” and a drop down list will appear. Select channel “FL1-A.”
- Highlight a well to analyze. Select “Gate” button, and manually draw a gate for the population of interest.
- Flow Plus will provide cell “Count” within this gate and “% of This Plot”.
Representative Results
In this system, a glucose-dependent change in glycosome composition was observed. When cells are grown in glucose containing media, two populations are observed; one bright and one dim (Figure 2A). Dim cells harbor immature glycosomes, which have not imported the PTS2eYFP while bright cells harbor a mixture of mature and immature glycosomes12. When glucose is present in the media, mislocalization of glycosome proteins is lethal15,28 and glycosome protein expression is likely coupled closely with import. This tight regulation is responsible for the appearance of dim cells in which glycosome protein expression is suppressed when the cells lack sufficient import capability. Once the glycosome import machinery is fully assembled and functional, the cells express glycosome proteins, which are then imported into glycosomes, yielding fluorescent cells. In glucose-deplete media, the mislocalization of glycosome proteins is tolerated15, and the bimodal population distribution is replaced by a single peak with a broader range of fluorescence (Figure 2B).
This system has been used to identify conditions that trigger changes in glycosome composition. When high density cultures containing ~10% dim cells (Figure 3A) are passed into fresh media, there is a transient increase in the percentage of dim cells (Figure 3B). By 24 hr. the original population distribution is reestablished (Figure 3C).
| SDM79 Solids | Weight (g/l) |
| Graces insect cell media powder | 2 |
| Glucose | 1 |
| HEPES | 8 |
| MOPS | 5 |
| NaHCO3 | 2 |
| Sodium pyruvate | 0.1 |
| L-Alanine | 0.2 |
| L-Arginine | 0.1 |
| L-Glutamine | 0.3 |
| L-Methonine | 0.07 |
| L-Phenylalanine | 0.08 |
| L-Proline | 0.6 |
| L-Serine | 0.06 |
| L-Taurine | 0.16 |
| L-Threonine | 0.35 |
| L-Tyrosine | 0.1 |
| Adenosine | 0.01 |
| Guanosine | 0.01 |
| Glucosamine HCl | 0.05 |
| Folic acid | 0.004 |
| r-Aminobenzoic acid | 0.002 |
| Biotin | 0.0002 |
Table 1. SDM79 solid components.Solid media components and amount (g/l) are provided.
| SDM79 | |
| MEM with glutamine | 600 ml |
| Pen/Strep | 10 ml |
| BME vitamin solution | 10 ml |
| MEM amino acids solution | 8 ml |
| MEM non essential amino acid solution | 6 ml |
| Hemin | 3.75 |
| Water | 162.25 ml |
Table 2.SDM79 liquid components.Volumes ml are given.
| Cytomix | For 20 ml |
| 120 mM KCl | 1.4 ml (1 M) |
| 0.15 mM CaCl2 | 3 ml (1 M) |
| 10 mM K2HPO4 | 400 ml (0.5 M) |
| 25 mM HEPES | 500 ml (1 M) |
| 2 mM EDTA | 80 ml (0.5 M) |
| 5 mM MgCl2 | 100 ml (1 M) |
| Water | 16.52 |
Table 3. Cytomix recipe.
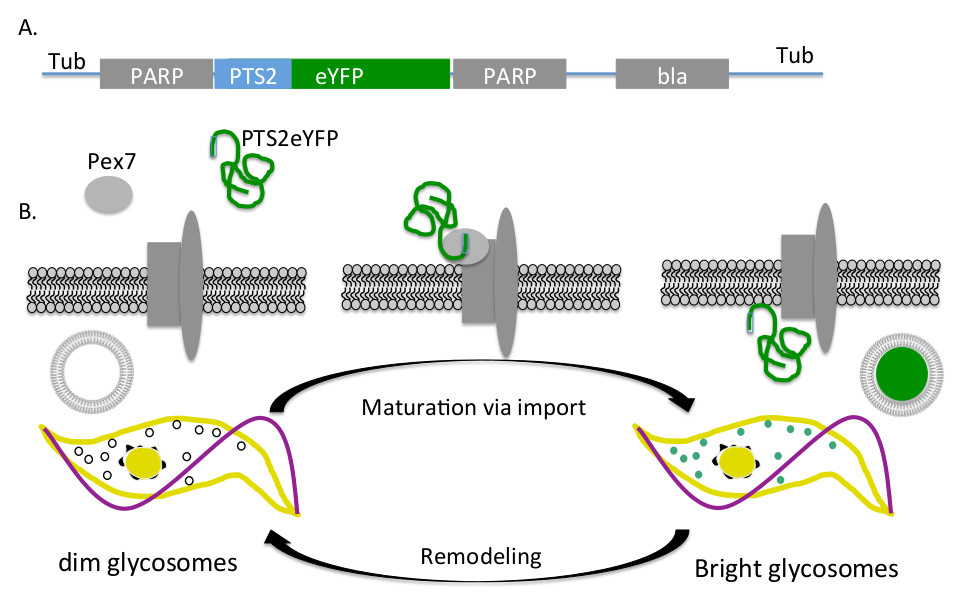
Figure 1. Fluorescent glycosome reporter system. A) PTS2eYFP expression construct integration. PCF trypanosomes were transformed with the pXS2PTS2eYFP plasmid linearized with the restriction enzyme MluI. This construct integrates via homologous recombination into the tubulin locus (Tub) and PARP sequences (PARP) includes the promoter that drives the constitutive expression of PTS2eYFP and the sequences required for RNA processing. B) PTS2eYFP import. PTS2eYFP is synthesized in the cytoplasm where it binds the soluble receptor, PEX7, which delivers the reporter protein to the glycosomes. Once delivered to the glycosome membrane, the protein is imported. Mature organelles containing import machinery import PTS2eYFP and fluorescence while those that do not contain functional import machinery remain dim.
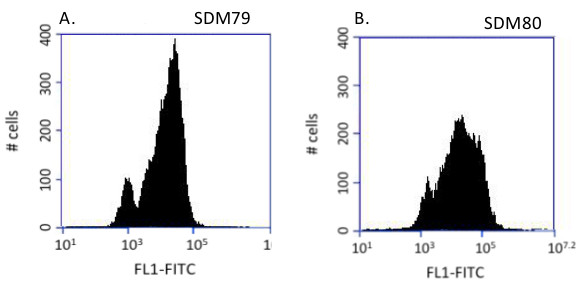
Figure 2. Glucose-dependent glycosome composition. PCF cells were grown in SDM79 (+Glc) or SDM80 (-Glc) and analyzed by flow cytometry. Histograms of 10,000 events. A) Analysis of cells grown in SDM79 consistently reveals two peaks. Fluorescent cells harbor a mixture of mature and immature organelles with cells of higher fluorescence intensities having more mature glycosomes. In SDM79, mislocalization of glycosome proteins is lethal and glycosome protein expression and import must be tightly controlled. This is reflected in the absence of cells with intermediate fluorescence intensities. B) In SDM80, the mislocalization of glycosome proteins is tolerated, the bimodal population distribution is lost, and cells of intermediate fluorescence are observed.
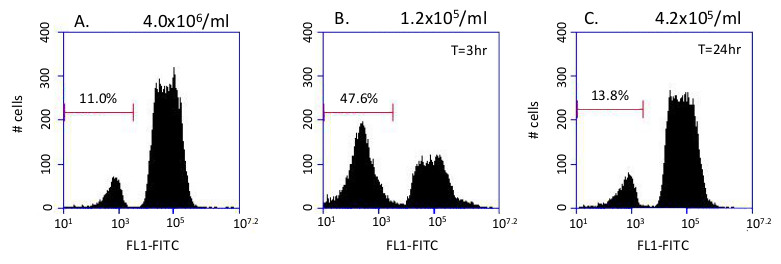
Figure 3. Glycosome remodeling. Remodeling of glycosomes is triggered by passage into fresh media. A) Histogram of 10,000 events in the starting culture (~4 x 106/ml). B) 3 hr after dilution into fresh SDM79 there is an increase in the proportion of dim cells with immature glycosomes falling within the left gate C) Histogram of the diluted culture after 24 hr. After 24 hr, the population distribution has returned to normal, as the immature glycosomes now contain the peroxisome proteins necessary to import the fluorescent protein.
Discussion
Glycosomes are essential, dynamic, parasite-specific organelles. The processes that regulate the biogenesis, maintenance, proliferation and remodeling of these organelles likely include drug targets that could be exploited for therapeutic purposes. Despite the potentially high abundance of such drug targets, the field of glycosome biogenesis has lagged behind the study of similar processes in other organisms, predominately due to the lack of a tractable, high-throughput system by which to monitor rapid, dynamic, organelle responses in live cells.
Fluorescent-organelle reporter systems have been used to study organelle dynamics in higher eukaryotes such as yeast, plants, fungi and mammals23-25. We have generated a transgenic strain of PCF parasites that express PTS2eYFP that is targeted to mature glycosomes, yielding fluorescent organelles. Changes in glycosome composition can be monitored via following cellular fluorescence. Using this system, we have found that environmental conditions, notably glucose, regulate glycosome composition12.
In contrast to microscopy methods often used to study organelle dynamics in kinetoplastid parasites, this reporter system offers a method by which to rapidly screen compounds and conditions that influence overall glycosome dynamics. However, the changes in fluorescence reflect changes in overall organelle compositions. Further biochemical and microscopic experiments are required to define the specific molecular differences between cell populations exhibiting different fluorescence intensities.
We have identified a number of critical steps in the remodeling assays. Interestingly, we have found that as the cells are cultured for longer periods (more than two passes), their behavior in response to environmental changes is unpredictable. After prolonged culturing, cells passed from high densities into fresh media, exhibit a temporary increase in the dim population, indicating that they are still able to remodel glycosomes, but die after 24 hr. We have also encountered situations where no remodeling is observed. The reason for this behavior is unclear but we have found that when cells are handled as described here (limiting the number of cell passages to two and maintaining cultures below 1 x 107/ml), glycosome remodeling is reproducible. When cells are no longer responsive to environmental conditions, retransforming cells with the reporter-construct plasmid usually remedies this situation.
While this system has been used to study glycosome behavior in African trypanosomes, it can also be adopted to the study of organelles in other parasites by fusing fluorescent proteins to amino acid sequences that direct protein localization to other cellular compartments.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by the Creative Inquiry Program for Undergraduate Research and the Calhoun Honors College at Clemson University.
Materials
| Adenosine | Avocado Research Chemicals Ltd | A10781 | SDM79 Ingredient |
| L-Alanine | Avocado Research Chemicals Ltd | A15804 | SDM79 Ingredient |
| L-arginine | CalBiochem | 1820 | SDM79 Ingredient |
| p-aminobenzoic acid | ICN Biomedicals | 102569 | SDM79 Ingredient |
| Basal Medium Eagle Vitamin Solution (100X) | Sigma | B6891 | SDM79 Ingredient |
| Biotin | Fisher | BP232-1 | SDM79 Ingredient |
| Calcium Chloride | VWR | BDH0224 | Cytomix |
| EDTA | Fisher | S311-100 | Cytomix ingredient |
| EZNA Gel Extraction kit | Omega Biotek | D2500-01 | DNA purifiation |
| Research grade Serum | Fisher | 03-600-511 | SDM79 Ingredient |
| Folic acid | ICN Biomedicals | 101725 | SDM79 Ingredient |
| Glucosamine HCl | ICN Biomedicals | 194671 | SDM79 Ingredient |
| Glucose | GIBCO | 15023-021 | SDM79 Ingredient |
| L-glutamine | CalBiochem | 3520 | SDM79 Ingredient |
| Glycerol | Acros Organics | Ac15892-0010 | Freezing media |
| Graces insect cell media powder | GIBCO | 11300-043 | SDM79 Ingredient |
| Hemin | MP Biomedicals | 194025 | SDM79 Ingredient |
| Guanosine | Avocado Research Chemicals Ltd | A11328 | SDM79 Ingredient |
| HEPES | MP Biomedicals | 194025 | SDM79 Ingredient |
| Magnesium Chloride | Fisher | BP214-500 | Cytomix ingredient |
| L-methionine | Fisher | BP388-100 | SDM79 Ingredient |
| MEM Amino Acids (50X) | Cellgro | 25-030-CI | SDM79 Ingredient |
| NEAA Mixture (100X) | Lonza | 13-114E | SDM79 Ingredient |
| Minimal Essential Medium (1X) with L-glutamine | Cellgro | 10-010-CM | SDM79 Ingredient |
| MOPS | Fisher | BP308-500 | SDM79 Ingredient |
| Sodium Biocarbonate | Fisher | S233-500 | SDM79 Ingredient |
| Penicillin-Streptomycin Solution | Cellgro | 30-002-CI | SDM79 Ingredient |
| L-phenylalanine | ICN Biomedicals | 102623 | SDM79 Ingredient |
| Potassium Chloride | Fisher | P217-500 | Cytomix ingredient |
| Potassium Phosphate Dibasic Anhydrous | Fisheer | P290-212 | Cytomix ingredient |
| L-proline | Fisher | BP392-100 | SDM79 Ingredient |
| L-serine | Acros Organics | 56-45-1 | SDM79 Ingredient |
| Pyruvic acid, sodium salt | Acros Organics | 113-24-6 | SDM79 Ingredient |
| L-taurine | TCI America | A0295 | SDM79 Ingredient |
| L-threonine | Acros Organics | 72-19-5 | SDM79 Ingredient |
| L-tyrosine | ICN Biomedicals | 103183 | SDM79 Ingredient |
| E.Z.N.A.Cycle Pure kit | Omega Biotek | D6492-02 | DNA purification |
| Binding buffer | Omega Biotek | PDR041 | DNA purification |
| SPW wash buffer | Omega Biotek | PDR045 | DNA purification |
| Gene Pulser Xcell | Biorad | 165-2660 | Trypanosome transformation |
| 4 mm electroporation cuvettes | VWR | Trypanosome transformation |
References
- Stuart, K., et al. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 118, 1301-1310 (2008).
- Herman, M., Perez-Morga, D., Schtickzelle, N., Michels, P. A. Turnover of glycosomes during life-cycle differentiation of Trypanosoma brucei. Autophagy. 4, 294-308 (2008).
- Herman, M., Gillies, S., Michels, P. A., Rigden, D. J. Autophagy and related processes in trypanosomatids: insights from genomic and bioinformatic analyses. Autophagy. 2, 107-118 (2006).
- Michels, P. A., Bringaud, F., Herman, M., Hannaert, V. Metabolic functions of glycosomes in trypanosomatids. Biochim Biophys Acta. 1763, 1463-1477 (2006).
- Michels, P. A., et al. Peroxisomes, glyoxysomes and glycosomes (review). Mol Membr Biol. 22, 133-145 (2005).
- Moyersoen, J., Choe, J., Fan, E., Hol, W. G., Michels, P. A. Biogenesis of peroxisomes and glycosomes: trypanosomatid glycosome assembly is a promising new drug target. FEMS Microbiol Rev. 28, 603-643 (2004).
- Parsons, M., Furuya, T., Pal, S., Kessler, P. Biogenesis and function of peroxisomes and glycosomes. Molecular and biochemical parasitology. 115, 19-28 (2001).
- Parsons, M. Glycosomes: parasites and the divergence of peroxisomal purpose. Mol Microbiol. 53, 717-724 (2004).
- Michels, P. A., Hannaert, V., Bringaud, F. Metabolic aspects of glycosomes in trypanosomatidae – new data and views. Parasitol Today. 16, 482-489 (2000).
- Michels, P. A., Hannaert, V. The evolution of kinetoplastid glycosomes. J Bioenerg Biomembr. 26, 213-219 (1994).
- Hannaert, V., Michels, P. A. Structure function, and biogenesis of glycosomes in kinetoplastida. J Bioenerg Biomembr. 26, 205-212 (1994).
- Bauer, S., Morris, J. C., Morris, M. T. Environmentally regulated glycosome protein composition in the african trypanosome. Eukaryot Cell. 12, 1072-1079 (2013).
- Colasante, C., Ellis, M., Ruppert, T., Voncken, F. Comparative proteomics of glycosomes from bloodstream form and procyclic culture form Trypanosoma brucei brucei. Proteomics. 6, 3275-3293 (2006).
- Opperdoes, F. R. Compartmentation of carbohydrate metabolism in trypanosomes. Annu Rev Microbiol. 41, 127-151 (1987).
- Kessler, P. S., Parsons, M. Probing the role of compartmentation of glycolysis in procyclic form Trypanosoma brucei: RNA interference studies of PEX14, hexokinase, and phosphofructokinase. The Journal of biological chemistry. 280, 9030-9036 (2005).
- Bringaud, F., Riviere, L., Coustou, V. Energy metabolism of trypanosomatids: adaptation to available carbon sources. Molecular and biochemical parasitology. 149, 1-9 (2006).
- Coustou, V., et al. Glucose-induced remodeling of intermediary and energy metabolism in procyclic Trypanosoma brucei. The Journal of biological chemistry. 283, 16342-16354 (2008).
- Lamour, N., et al. Proline metabolism in procyclic Trypanosoma brucei is down-regulated in the presence of glucose. The Journal of biological chemistry. 280, 11902-11910 (2005).
- Vertommen, D., et al. Differential expression of glycosomal and mitochondrial proteins in the two major life-cycle stages of Trypanosoma brucei. Molecular and biochemical parasitology. 158, 189-201 (2008).
- Vickerman, K. Developmental cycles and biology of pathogenic trypanosomes. British medical bulletin. 41, 105-114 (1985).
- Lorenz, P., Maier, A. G., Baumgart, E., Erdmann, R., Clayton, C. Elongation and clustering of glycosomes in Trypanosoma brucei overexpressing the glycosomal Pex11p. The EMBO journal. 17, 3542-3555 (1998).
- Saveria, T., et al. Conservation of PEX19-binding motifs required for protein targeting to mammalian peroxisomal and trypanosome glycosomal membranes. Eukaryot Cell. 6, 1439-1449 (2007).
- Banerjee, S. K., Kessler, P. S., Saveria, T., Parsons, M. Identification of trypanosomatid PEX19: functional characterization reveals impact on cell growth and glycosome size and number. Molecular and biochemical parasitology. 142, 47-55 (2005).
- Milagros Camara Mde, L., Bouvier, L. A., Miranda, M. R., Pereira, C. A. Identification and validation of Trypanosoma cruzi’s glycosomal adenylate kinase containing a peroxisomal targeting signal. Experimental parasitology. 130, 408-411 (2012).
- Wiemer, E. A., Wenzel, T., Deerinck, T. J., Ellisman, M. H., Subramani, S. Visualization of the peroxisomal compartment in living mammalian cells: dynamic behavior and association with microtubules. The Journal of cell biology. 136, 71-80 (1997).
- Kim, P. K., Mullen, R. T., Schumann, U., Lippincott-Schwartz, J. The origin and maintenance of mammalian peroxisomes involves a de novo PEX16-dependent pathway from the ER. The Journal of cell biology. 173, 521-532 (2006).
- Hoepfner, D., Schildknegt, D., Braakman, I., Philippsen, P., Tabak, H. F. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 122, 85-95 (2005).
- Furuya, T., et al. Glucose is toxic to glycosome-deficient trypanosomes. Proc Natl Acad Sci U S A. 99, 14177-14182 (2002).

