Transferring Cognitive Tasks Between Brain Imaging Modalities: Implications for Task Design and Results Interpretation in fMRI Studies
Summary
Transferring a paradigm with a history of use in EEG experiments to an fMRI experiment is considered. It is demonstrated that manipulating the task demands in the visual oddball task resulted in different patterns of BOLD activation and illustrated how task design is crucial in fMRI experiments.
Abstract
As cognitive neuroscience methods develop, established experimental tasks are used with emerging brain imaging modalities. Here transferring a paradigm (the visual oddball task) with a long history of behavioral and electroencephalography (EEG) experiments to a functional magnetic resonance imaging (fMRI) experiment is considered. The aims of this paper are to briefly describe fMRI and when its use is appropriate in cognitive neuroscience; illustrate how task design can influence the results of an fMRI experiment, particularly when that task is borrowed from another imaging modality; explain the practical aspects of performing an fMRI experiment. It is demonstrated that manipulating the task demands in the visual oddball task results in different patterns of blood oxygen level dependent (BOLD) activation. The nature of the fMRI BOLD measure means that many brain regions are found to be active in a particular task. Determining the functions of these areas of activation is very much dependent on task design and analysis. The complex nature of many fMRI tasks means that the details of the task and its requirements need careful consideration when interpreting data. The data show that this is particularly important in those tasks relying on a motor response as well as cognitive elements and that covert and overt responses should be considered where possible. Furthermore, the data show that transferring an EEG paradigm to an fMRI experiment needs careful consideration and it cannot be assumed that the same paradigm will work equally well across imaging modalities. It is therefore recommended that the design of an fMRI study is pilot tested behaviorally to establish the effects of interest and then pilot tested in the fMRI environment to ensure appropriate design, implementation and analysis for the effects of interest.
Introduction
As cognitive neuroscience methods develop, established experimental tasks are used with emerging brain imaging modalities. This is a logical progression since most neuropsychological concepts (e.g., distinct memory sub-components) have been investigated in the behavioral domain and appropriate experimental tasks for probing specific functions have been developed and tested. As new technology emerges evidence for the neural underpinnings of these behavioral observations is sought with the new brain imaging methods. While it may be tempting to simply draw on well-studied behavioral tasks for imaging studies, several important caveats have to be taken into account. One crucial, though frequently neglected, consideration is the use of the most appropriate imaging technique to further probe the behavioral evidence. In terms of cognitive neuroscience and psychology there are many brain imaging methods available to enhance our understanding of the neural activity underlying concepts of interest; for example electroencephalography (EEG), magnetoencephalography (MEG), transcranial magnetic stimulation (TMS), functional magnetic resonance imaging (fMRI) and positron emission tomography (PET). All of these methods have their advantages, disadvantages and appropriate applications. Here transferring a paradigm with a long history of behavioral and EEG experiments to an fMRI experiment is considered. EEG has been used for decades to investigate neural responses associated with perceptual and cognitive processes. As such, many paradigms have been developed for use with this method and have evolved over time. Functional MRI is a technique that emerged more recently in cognitive neuroscience and this has led to some paradigms developed in EEG research being used in fMRI. To build on the knowledge base from EEG experiments with the new techniques is a logical step but nonetheless some important points may be neglected in the transfer. The techniques are very different and tasks need to be designed accordingly. This requires knowledge of how the method works and, in particular, how potential modulations of the paradigm used will influence the measures taken. For further information on the design of fMRI experiments the interested reader is directed to the following link http://imaging.mrc-cbu.cam.ac.uk/imaging/DesignEfficiency. Task design will be considered in the context of transferring a paradigm developed for EEG research to the fMRI environment. The aims of this paper are: i) to briefly describe fMRI and when its use is appropriate in cognitive neuroscience; ii) to illustrate how task design can influence the results of an fMRI experiment, particularly when that task is borrowed from another imaging modality; and iii) to explain the practical aspects of performing an fMRI experiment.
Functional MRI is now a widely available technique and as such is a common method used in cognitive neuroscience. In order to make a decision as to whether the technique is appropriate for a particular experiment the advantages and disadvantages of fMRI must be considered in relation to other available techniques. A disadvantage of the method is that it is not a direct measure of neural activity, rather it is a correlate of neural activity in that the metabolic response (oxygen requirement) convolved with the haemodynamic response. Thus its temporal resolution is poor in comparison to electrophysiology, for example, where the measured electrical signal is closer to the underlying neural activity rather than a metabolic response. EEG has a temporal resolution in the order of milliseconds compared to a resolution in the order of seconds in fMRI. However, the main advantage of fMRI is that the spatial resolution of the technique is excellent. Furthermore, it is noninvasive and thus subjects do not have to ingest substances such as contrast agents or be exposed to radiation as would be the case in positron emission tomography (PET). Therefore, fMRI is a suitable technique for experiments investigating which brain regions are involved in perception, cognition, and behavior.
In this paper the visual oddball paradigm is taken as an example for the transfer of a well-established EEG-task to fMRI (see Figure 1 for details). It should be noted that the issues discussed could also influence results and data interpretation when other paradigms are used and should technically be considered in the design of all fMRI experiments. The oddball paradigm is frequently used in psychology and cognitive neuroscience to assess attention and target detection performance. The paradigm was developed in EEG research, specifically event related potentials (ERPs), for investigating the so called P300 component1. The P300 represents target detection and is elicited upon recognition of an infrequent target stimulus1. The P300 is used in studies across a number of cognitive and clinical domains2 e.g., patients with schizophrenia and their relatives3, heavy smokers4 and the aging population5. Given that the oddball paradigm (and the P300 elicited by the paradigm) is robust and is also modulated by different disease states, its transfer across different imaging modalities was inevitable.
The widespread activation seen in the brain during an oddball fMRI measurement is known to be the result of multiple cognitive functions, as shown by numerous fMRI studies probing other cognitive concepts. This widespread nature of the activation pattern makes it difficult to determine which brain regions are more (or less) active due to the specific task manipulations or group differences that the experimenter is interested in. Specifically, it is not certain whether observed differences in activation are related to target detection itself, to attention related processes, or whether they are related to other task demands such as ongoing working memory processes or processes related to the production of a motor response. The process of assigning function to the measured activity is easier in the EEG domain where the cognitive component of interest (target detection) is measured in clear cerebral response to the oddball task (P300). Nevertheless, neuroscientists tend to interpret their findings in favor of their own hypothesis and experiment, rather than putting in the effort to rule out alternative explanations. Most experiments, however, will not be able to solve these important questions inherently – scan time is costly – which is why we argue for thorough planning and pilot testing of paradigms.
Besides this difficulty in establishing a direct link between brain regions and cognitive components, the nature of the oddball paradigm also presents other possible methodological issues when being transferred to fMRI. For example, the detection of a target stimulus is usually indicated by pressing a response button. This allows the experimenter to record the accuracy and speed of responses but this response may also impact on the fMRI BOLD response to target stimuli. The motor action required for the button press impacts on stimulus-locked fMRI activation given that it happens just a few hundred milliseconds after the presentation of the target stimulus. This can also influence interpretation of that activation, for example brain regions involved in preparing for the motor response might wrongly be assumed to be involved in the detection of the target stimulus, and vice versa. This has led to methodological modifications whereby indirect measures of target detection, not relying on motor responses, are taken. For example, counting target stimuli has been proposed6 as a way to make sure subjects maintain attention on the task; the number of trials missed can indicate how inattentive a subject was. Reporting the number of stimuli counted at the end of the task also means that the experimenter can check whether the subject performed the task correctly. A third alternative is to use a fully passive task design where the subject is given no instructions on how to respond and the novelty of a target stimulus is assumed to inherently elicit a target detection-like response. Despite these versions of the task using the same type of stimuli and basic design, the activation pattern resulting from each variation of the task will be different because the cognitive and motor demands of the tasks are different7,8. For example, there will be working memory processes involved in counting target stimuli e.g., holding the current number of target stimuli in mind, that will not be required during passive viewing. Here these 3 versions of the oddball task, passive, count ,and respond are used to show how careful task design and implementation can account for these changes in task requirements and allow appropriate interpretation of the results.
Protocol
NOTE: The study protocol was approved by the local Human Subjects Review Board at RWTH Aachen University and was carried out in accordance with the Declaration of Helsinki.
1. Task Design
- Choose an appropriate task to investigate the cognitive/psychological construct of interest. Use the visual oddball task (Figure 1) to measure target detection responses and the effects of attention on target detection. This allows investigation of the influence of task manipulations on fMRI data.
- Use three versions of the oddball task.
- Passive version: Ask the subject to observe visual stimuli. Do not detect any response.
- Silent count version: Ask the subject to count the target stimuli. This task requires directing attention towards these stimuli and a discriminating process.
- Respond version: Ask the subject to push a response button upon seeing a target stimulus. This task requires attention, discrimination processes, and selection/production of a response to target stimuli.
- Consider the appropriate number of trials required for a robust response. The signal to noise ratio in fMRI measurements is relatively low and requires a number of responses to be averaged in order to investigate the effects of interest9. This depends on the task and stimulus modality used. 200 trials are used in this task, 40 of which are target trials sufficient to elicit a robust response.
- Determine the timings for the sequence of stimuli. The timing of stimuli is crucial in an fMRI study for consideration of presentation rate10. Consider the hemodynamic response-delay between stimulus onset and the measured brain response (Figure 2).
- Maintain a balance between delivering sufficient stimuli in a reasonable amount of time and allow sufficient sampling of the hemodynamic response to each stimulus, including the return to baseline. Download, install and run optseq software. Run optseq to optimally distributing the trials across the experiment based on number of trials, stimulus duration and scanning parameters (repetition time and number of volumes).
- Implement the order of stimuli (previously determined) in a suitable program for presenting the paradigm to the subject.
- Specify all information relevant to the paradigm in terms of type of stimuli, timing and responses.
NOTE: Programming details are not presented here because each paradigm will have different requirements as will different software packages.
- Specify all information relevant to the paradigm in terms of type of stimuli, timing and responses.
- Set up the program that will deliver the experimental paradigm so that it will start with a trigger from the scanner. This allows synchronization of the acquired data and the sequence of stimuli presented.
2. Setup Experimental Environment
- Prepare the scanner room. Connect the bottom part of correct head coil to the scanner bed. Place clean protective covers on the scanner bed and cushions.
- Use a display device to present the experimental paradigm to the subject and record the responses using a hand held device. Switch the display device and a hand held device “on”.
- Start the software that will deliver the experimental paradigm and provide a name for the logfile. The logfile contains information about the timing of the stimuli and of the responses made by the subject. Use this information for analyzing the data.
- Register the subject in the MR scanner database. Record data using a unique identification number. Do not store subject’s name with the data to guarantee privacy.
- Make sure that the MR sequences to be run are set up and ready. Use the following sequences: a localizer scan to obtain the subjects’ head position inside the coil, an EPI sequence for the functional imaging and MPRAGE for a high resolution structural scan.
3. Subject Arrival and Entrance to Scanner
- Screen the subject for contraindications with MRI prior to the experiment (e.g., during the recruitment procedure).
- Provide MR safety instructions before scanning. Perform screening of subjects (by trained personnel). Ensure subjects’ safety. Make sure they have no metal in their body, do not have devices such as pacemakers and do not meet any other exclusion criteria.
- Upon subjects’ arrival check the screening questionnaire and confirm their compatibility before proceeding.
- Explain the experimental procedure to the subject and offer the opportunity to ask questions. Ask the subject to sign the consent and data protection forms.
- If the experiment involves complex tasks requiring training it is recommended that the subject performs a practice run prior to going in the scanner.
- Ensure that the subject is metal free, without any coins, belt, watch and jewelry. Once confirmed, let the subject in the scanner room.
- Ask the subject to sit on the scanner bed wearing earplugs. The earplugs used here provide protection against the noise from the scanner during scanning and also allow the investigator to communicate directly with the subject from the control room. In some facilities headphones are used for communication with the subject.
- Ask the subject to lie down on the scanner bed. Offer the subject a cushion to go under the knees to reduce back pain. The comfort of the subject is important for their well-being and data quality. Movement resulting from discomfort will have a negative impact on the imaging data and distraction caused by discomfort will influence the performance of the task.
- Place the top part of the head coil over the subject’s head and plug in the connectors. Position the subject’s head appropriately in the head coil. Align the small marker on the head coil along the subjects’ eyebrows. Ensure the subject is lying straight and comfortable. The coil surface should not touch the face (e.g., pressing on the nose).
- Secure the subject’s head with small cushions to minimize head movements during scanning. Head movements have negative impact on the quality of the data.
- Place a mirror on top of the head coil for the subject to see the experimental paradigm displayed on the screen behind. Ensure the subject can see the whole screen. Move the mounted mirror according to the subject’s position. Subjects with glasses must wear MR compatible eyewear. Most MRI research facilities have compatible lenses or goggles. In this case, mount MR compatible lenses on the frame that holds the mirror. Determine the appropriate lens strength before the subject enters the scanner room.
- Hand the subject an emergency call button to stop the scan if required. Make sure the subject knows where the button is and that they can easily reach it.
- Move the subject to the entrance of the bore of the scanner. Ask the subject to close their eyes during this procedure. Align the light with the small marks on the head coil to establish the correct position.
- Move the subject into the bore of the scanner until the display reads ‘0 mm’. This means that the head of the subject is at the isocenter of the scanner.
- Hand the subject the response device.
4. Experimental Procedure
- Check if the subject can hear the experimenter via the intercom and that the subject is comfortable and ready to start.
- Perform a localizer scan to obtain the position of the subject’s head in the scanner. Use this to position the field of view of all of the remaining measurements to determine the parts of the brain to be measured.
- First perform a high resolution structural scan. Open the MPRAGE sequence/program and position the field of view. Ensure the entire subject’s head is within the field of view. MP-RAGE parameters: TR/TE = 2,250 / 3.03 msec, flip angle = 9°, 176 sagittal slices, FOV 256 x 256 mm, 64 x 64 matrix, voxel size 1 x 1 x 1 mm).
- Let the subject know that the scan will start and then begin the measurement.
- Perform functional MRI scan.
- Open the EPI sequence on the scanner computer and align the field of view to cover the entire brain. EPI parameters: 33 slices, slice thickness 3 mm, FOV 200 x 200 mm, 64 x 64 matrix, repetition time 2,000 msec, echo time 30 msec, flip angle 79°.
- Run a single volume test measurement. Make sure that the whole (or as much as possible) of the subject’s brain is contained within the field of view.
NOTE: Subjects have different shapes and sizes of heads (and brains). Hence, optimally position the field of view for each subject. - Copy the fMRI sequence so that the positioned field of view remains the same for the next measurement. Enter the number of volumes required for the measurement, 304 in this case.
- Make sure the software presenting the paradigm is waiting for a trigger from the scanner. The paradigm will not start without a trigger from the scanner so it can be loaded and set to wait.
- Inform the subject that the experiment is about to start. Start the measurement.
- Check that the software presenting the paradigm starts at the appropriate time (i.e. that it is triggered by the scanner).
- Perform three versions of the oddball task. Passive, Count, and Respond.
- Speak to the subject in between runs to provide reassurance. Ensure their comfort. Ask if the subject permits to continue with the study. Instruct the subject of the upcoming task.
- First run the passive condition to ensure true passive viewing without knowledge that the target stimuli are indeed target stimuli. Counterbalance the order of the count and respond conditions across subjects to prevent order effects.
5. End of Experiment
- Inform the subject that the experiment is finished enter the scanner room.
- Slide the subject out of scanner.
- Remove head coil and cushions.
- Ask the subject to sit up slowly. Once they are comfortable, the subject can stand up and leave the scanner room.
- Administer any questionnaires/paperwork that needs to be completed after the experiment
- Debrief the subject: provide the subject with an explanation about the aims and purpose of the study if this was not fully possible prior to the experiment and offer the opportunity to ask questions
6. Data Analysis
- Use a software package that is suitable for analyzing fMRI data. Perform first level data analysis for each subject and each condition separately.
NOTE: Use the FMRIB Software Library (FSL) for fMRI data analysis. - Apply standard preprocessing steps to prepare the data for further analysis.
NOTE: Apply the following steps: motion correction, slice timing correction, coregistration of structural and function data, spatial smoothing, highpass temporal filtering, normalization of individual into standard (e.g., MNI) space. Find a summary of these steps in fMRI textbooks Huettel et al, (2008)9 and Jezzard et al, (2001)11. Specific information on how to perform preprocessing steps is available on the website and in the supporting documentation for each individual software package. - For the statistical analysis specify the onset times and durations of all events. These are termed explanatory variables (EVs), or regressors.
- Set up contrasts to determine which EVs are compared. To identify the BOLD activation specific to detection of target stimuli set up the following contrast: target > non-target stimuli.
NOTE: Optionally use other contrasts: target stimuli against baseline; non-target stimuli against baseline; target stimuli > non-target stimuli; non-target stimuli > target stimuli - Perform the first level statistical analysis for each subject and each condition separately. The output of the analysis shows the active brain regions for each of the respective contrast.
- Compare the three conditions using a second level, or group level, analyses. Use the output of the first level analysis as the input for the group level analysis.
NOTE: In the original paper7 the differences between conditions on the target > frequent contrast using a Tripled Two-Group Difference design involving the following contrasts: respond > passive, count > passive, respond > count. These contrasts reveal brain activity associated with the variation in cognitive processes across the three response modalities.
Representative Results
The stimulation and analysis method elicited BOLD activation in brain regions associated with a visual oddball task. The target > non-target contrast revealed no activation for the passive condition but did reveal activation in both the count and respond (Figure 3). The data presented in Figure 3 is a qualitative comparison of the count and respond conditions and shows how the activation patterns would look if each version of the task was performed in isolation.
The main comparisons of interest were those between the conditions. So, in which brain regions does target detection related activation differ when the task demands are changed? Figure 4 shows that there are differences between the conditions. In contrast to the qualitative assessment of the differences between the count and respond condition describe above, this comparison is done using t-tests on whole brain data, showing the regions where activation significantly differs between conditions.
The data from the original study7 show differences in BOLD activation between the count and respond versions of the visual oddball. If there was not data from both conditions for comparison the activation would be attributed to ‘target detection’ in both conditions. However, activation was observed in the middle frontal gyrus (MFG) during the respond but not the count condition. The fact that MFG activation was not observed in the count condition indicates that it is related to the motor preparation and/or motor response associated with the button press in the respond condition rather than purely to the target detection processes. In the absence of the count task for comparison it is likely that this MFG activation would have been attributed to cognitive processes associated with the task rather than action execution. Similarly, activation in the supplementary motor area (SMA) was observed during the count condition as well as the respond condition. There are no responses made in the count condition, so it is unlikely that the SMA activation is related to motor preparation, suggesting that the SMA plays a role in other aspects of the task such as attention to the stimuli, detection of target stimuli, deciding whether to make a response and if so which response to make. It is likely that SMA activation would have been interpreted as being involved in motor preparation if there was only a respond version of the task, meaning that the role of the SMA in other task related processes would have been overlooked. This highlights some potential pitfalls when interpreting fMRI data. Despite the task used here being relatively simple it involves many perceptual and cognitive processes. It can be difficult to differentiate these cognitive processes and their underlying neural substrates. The design of this study, allowing within scan assessment of the target detection contrast followed by between scan comparison of the conditions is a robust design, but it is not able to differentiate the possible roles of the SMA further than establishing that it contributes to processes other than motor processes. This highlights the need for careful experimental design and analysis in fMRI studies.
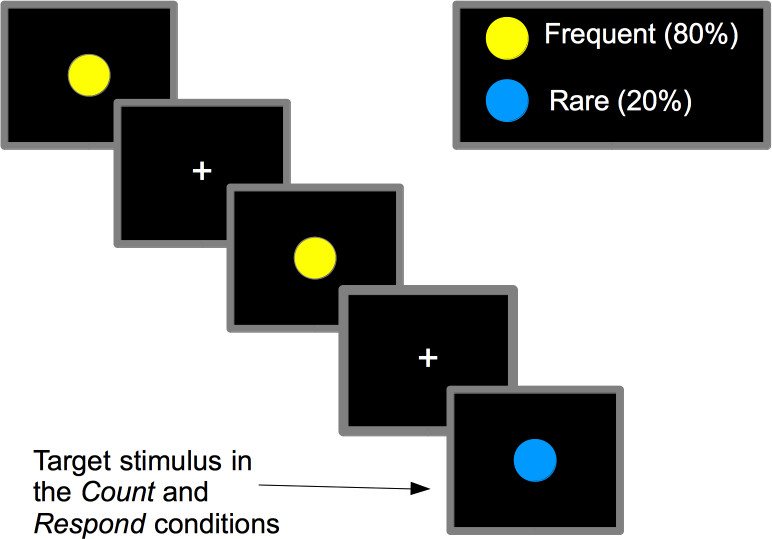
Figure 1. The oddball paradigm involves viewing a series of stimuli (in this case circles), 80% of which are of one type, ‘frequent’, and 20% are of a different type ‘target’. The target stimuli elicit a target detection response due to the infrequency of this type of stimulus. In this paper, 3 versions of the task were performed. The first is passive which involves passive viewing of the stimuli (no response made). The second is count, this involves counting the number of target stimuli and reporting the total at the end of the experiment. The third is respond, this involves pressing a button every time a target stimulus is displayed.
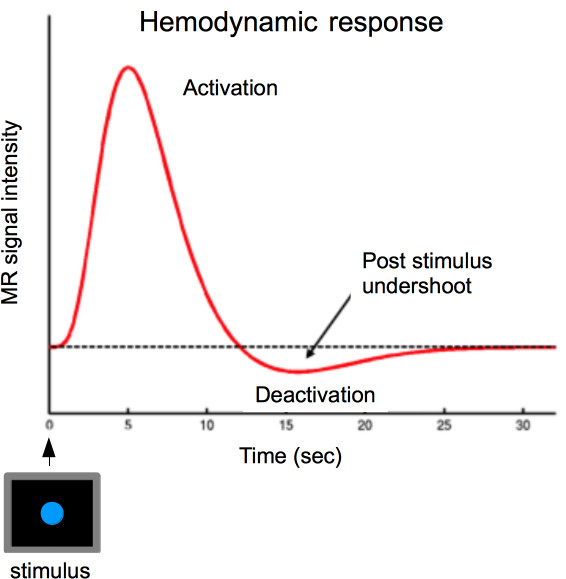
Figure 2. The hemodynamic response is the delivery of blood to neurally active tissues. The hemodynamic response in the brain rises slowly (in comparison to neural activity) and peaks approximately 5 sec after a stimulus. The response then takes a number of seconds (15-20) to return to baseline. The figure shows the canonical hemodynamic response function; this is a hypothetical signal in response to a single, short ‘zero duration’ stimulus, with the signal returning to baseline only if the stimulus no longer persists.
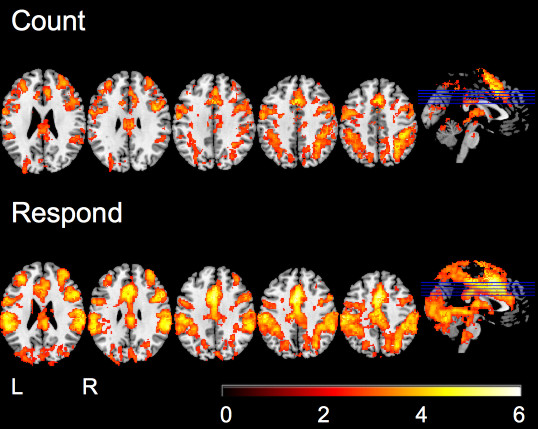
Figure 3. BOLD activation for the target > frequent contrast for the count and response conditions. (second-level mixed-effects FLAME. N = 16, Cluster-corrected threshold Z = 2.3, p = 0.05). This figure and caption have been modified from Warbrick et al, 20137.
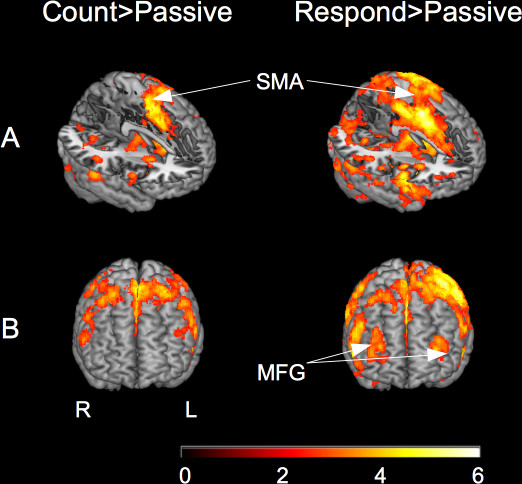
Figure 4. The left part of the figure shows BOLD activation for the count condition against the passive condition. The right part of the figure shows the respond condition against the passive condition. All data represent the target > frequent lower level contrast. Part A highlights activation in the supplementary motor area (SMA). Part B shows the middle frontal gyrus (MFG) activation for the respond condition only. (second-level mixed-effects FLAME. N = 16, Cluster-corrected threshold Z = 2.3, p = 0.05) This figure and caption have been modified from Warbrick et al, 20137.
Discussion
We show that manipulating the task demands in the visual oddball task results in different patterns of BOLD activation in the count and respond conditions. The functional roles of some of the regions implicated in each condition would have been inappropriately assigned had data from the three versions of the task not been available for comparison. This ambiguity in data interpretation would not necessarily have been the case in the EEG P300 field where the task has its origin, highlighting the need for special consideration when transferring experimental paradigms from one imaging modality to another. For example, many cognitive processes (such as attention and working memory) contribute to the generation of the P300 component but these are represented by a single electrophysiological marker, in contrast to the widespread activation seen in the fMRI BOLD response. Furthermore the P300 is not influenced by the motor response in the same way as fMRI data. The temporal resolution of EEG data allows cognitive and motor responses to be separated in time. The nature of the fMRI BOLD measure means that many brain regions are found to be active at the same time in a particular task. Determining the functions of these areas of activation is very much dependent on task design and analysis. It is therefore recommended that the design of an fMRI study is pilot tested behaviorally to establish the effects of interest and then pilot tested in the fMRI environment to ensure appropriate design, implementation and analysis for the effects of interest.
In addition to guiding the interpretation of data from oddball tasks involving a motor response the findings from the original study7 show that it is possible to design studies using the oddball task to focus on specific aspects of target detection. For example investigating the integration of sensory input to produce the correct motor response could be done using the respond version of the task. The count version of the task on the other hand would be more appropriate for investigating processes associated with decision making, specifically when a motor response is not required. In some populations, e.g., aging or patients with movement disorders, the production of a motor response might be affected by non-task related factors, in these cases the count version of the oddball task might be the most appropriate.
The data not only provide evidence for how brain activation patterns differ across versions of the oddball task, they also illustrate that considering the elements of cognitive/behavioral tasks used in fMRI experiments is crucial if the data are to be interpreted appropriately. This is particularly important in paradigms where it is possible to use an overt or covert response. Including a motor response changes the demands of the task and activation elicited by the motor response can influence the interpretation of other task related activation. Issues such as this should be considered when adapting a paradigm across different imaging modalities.
Disclosures
The authors have nothing to disclose.
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Magnetom Tim Trio 3T MRI scanner | Siemens Medical Solutions, Erlangen, Germany | ||
| Presentation version 14.8 | Neurobehavioural system, Albany, CA, USA | ||
| Lumitouch device | Photon Control Inc, Burnaby, BC, Canada | This device is no longer produced by the manufacturer. Alternative MR compatible response devices are available | |
| TFT display | Apple, Cupertino, CA, USA | 30inch cinema display | The screen was custom modified in-house to be MR compatible. However, a number of MR compatible screens are available on the market |
| optseq | surfer.nmr.mgh.harvard.edu/optseq | program for determining optimal stimulus timing for rapid event related designs | |
| FMRIB software library (FSL) | FMRIB, Oxford | http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/ | Other software tools are available for analysing fMRI data, for example SPM, AFNI and Brain Voyager |
References
- Squires, N. K., Squires, K. C., Hillyard, S. A. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and clinical neurophysiology. 38, 387-401 (1975).
- Polich, J., Criado, J. R. Neuropsychology and neuropharmacology of P3a and P3b. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 60, 172-185 (2006).
- Turetsky, B. I., et al. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophrenia bulletin. 33, 69-94 (2007).
- Mobascher, A., et al. The P300 event-related potential and smoking–a population-based case-control study. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 77, 166-175 (2010).
- Li, L., Gratton, C., Fabiani, M., Knight, R. T. Age-related frontoparietal changes during the control of bottom-up and top-down attention: an ERP study. Neurobiology of aging. 34, 477-488 (2013).
- Kirino, E., Belger, A., Goldman-Rakic, P., McCarthy, G. Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: An event-related functional magnetic resonance imaging study. Journal of Neuroscience. 20, 6612-6618 (2000).
- Warbrick, T., Reske, M., Shah, N. J. Do EEG paradigms work in fMRI? Varying task demands in the visual oddball paradigm: Implications for task design and results interpretation. Neuroimage. 77, 177-185 (2013).
- Warbrick, T., Arrubla, J., Boers, F., Neuner, I., Shah, N. J. Attention to Detail: Why Considering Task Demands Is Essential for Single-Trial Analysis of BOLD Correlates of the Visual P1 and N1. J Cogn Neurosci. 26, 529-542 (2014).
- Huettel, S. A., Song, A. W., McCarthy, G. . Functional magnetic resonance imaging. , (2008).
- Miezin, F. M., Maccotta, L., Ollinger, J. M., Petersen, S. E., Buckner, R. L. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 11, 735-759 (2000).
- Jezzard, P., Matthews, P. M., Smith, S. . Functional Magnetic Resonance Imaging: An Introduction to Methods. , (2001).

