The Attentional Set Shifting Task: A Measure of Cognitive Flexibility in Mice
Summary
The goal of this protocol is to perform a behavioral assay such as the attentional set shifting task (AST) to assess prefrontal cortex-mediated cognitive flexibility in mice.
Abstract
Cognitive impairment, particularly involving dysfunction of circuitry within the prefrontal cortex (PFC), represents a core feature of many neuropsychiatric and neurodevelopmental disorders, including depression, post-traumatic stress disorder, schizophrenia and autism spectrum disorder. Deficits in cognitive function also represent the most difficult symptom domain to successfully treat, as serotonin reuptake inhibitors and tricyclic antidepressants have only modest effects. Functional neuroimaging studies and postmortem analysis of human brain tissue implicate the PFC as being a primary region of dysregulation in patients with these disorders. However, preclinical behavioral assays used to assess these deficits in mouse models which can be readily manipulated genetically and could provide the basis for studies of new treatment avenues have been underutilized. Here we describe the adaptation of a behavioral assay, the attentional set shifting task (AST), to be performed in mice to assess prefrontal cortex mediated cognitive deficits. The neural circuits underlying behavior during the AST are highly conserved across humans, nonhuman primates and rodents, providing excellent face, construct and predictive validity.
Introduction
The attentional set shifting task (AST) was developed as a measure of attention and cognitive flexibility in rats over a decade ago1,2. AST is modeled after the intradimensional /extradimensional component of the Cambridge Neuropsychological Test Automated Battery (CANTAB) which is used to identify cognitive dysfunction in humans and non-human primates3,4. While the ability to learn simple rules remains intact, deficits in learning to modify a response when the rules have changed are found in patients suffering from a variety of neuropsychiatric disorders (i.e., schizophrenia, obsessive compulsive disorder, depression), neurodegenerative disorders (i.e., Parkinson’s disease) and in patients with lesions to the pre-frontal cortex5. More generally, these patients are described as having deficits in cognitive flexibility. Analogous deficits have been shown in both non-human primates and rodents when lesions to the prefrontal cortex have been induced1,6-8. These deficits indicate a state of cognitive inflexibility or an impaired ability to shift attentional set.
An attentional set is formed when a subject learns that a set of rules can be applied to complex stimuli in order to differentiate relevant from irrelevant cues. For example, in the AST, animals will learn to pay attention and respond to the relevant cue (i.e., digging medium) and ignore an irrelevant cue (i.e., odor), by pairing a food reward with the medium. This association is then reinforced in subsequent tasks where the type of digging medium and odor changes, but the paired association between medium and reward remains. This reinforced rule forms a cognitive set. Two stages within the AST protocol measure aspects of cognitive flexibility: reversal and the extra-dimensional shift. At the reversal stage, the previously negative stimuli within one dimension (medium in this example) is now positive, which challenges the animal to ignore the positive stimuli from the previous stage. For example, if felt digging medium was the positive stimuli in the previous stage and paper was the negative stimuli, now the reverse is true. This challenges the animal’s flexibility in that it must maintain the attentional set (i.e., medium is the relevant dimension) while altering the rule learned for stimulus and reward pair within a dimension. The formation of an attentional set is challenged at the extra-dimensional shift stage, when the irrelevant dimension (odor in this example) becomes the relevant dimension. A perseverative response, as indicated by a continued choice using the previously learned rule, at either stage reflects a deficit in cognitive flexibility.
Lesion studies in both non-human primates and rodents have shown specific regions of the prefrontal cortex can be attributed to the ability to perform particular stages of the AST1,9. Lesions to the orbitofrontal cortex (OFC), a subregion of the prefrontal cortex (PFC), have been shown to induce deficits in reversal learning on the AST8. Additionally, lesions to the medial prefrontal cortex (MPFC) lead to specific difficulties in performing the extra-dimensional shift.
While numerous studies using the AST have been performed in rats and non-human primates, relatively few have utilized AST as a measure of cognitive function in mice. Given the ease of genetic manipulation in mice, and the critical need to measure prefrontal cortex function in studying a variety of disorders, adapting and validating this behavioral measure in mice is an important addition to research on diseases associated with PFC dysfunction. The protocol detailed here is a modification of the procedure by Birrell and Brown1 that is optimized for application in mice and reflects many of the mouse-specific adaptations that have been previously reported2,6,10-13.
Protocol
NOTE: Animals used in this study are male C57Bl/6J mice between 4 and 6 months of age for optimal results. Mice are maintained on a reverse light cycle in order to conveniently test them during the active (dark) phase. If behavior is performed in a room separate from housing, mice are allowed at least 1 hr to acclimate following transportation to the testing room. Behavioral testing is performed under red light conditions minimize disruption to the normal activity of the mice as little as possible while still allowing the experimenter adequate illumination to visually monitor the testing session.
NOTE: All aspects of the experimental procedure described herein were carried out in accord with the Guide for the Care and Use of Laboratory Animals, 8th edition (NRC) and were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio.
1. Experimental Setup
- Fill ceramic ramekins (non-porous; 1.5” depth x 2.5' diameter), to inner rim with digging medium (approximately 1” from bottom); filter paper rounds cut in half and taped to inside back of ramekin (Figure 1A)
- Break lightly sweetened dry breakfast cereal (food reward) – Honey Nut or sugar coated varieties, into approximately 20 mg pieces for acclimation, training and testing stages.
- Make an attentional set shifting task (AST) chamber (Figure 1B): Acclimate train and test each mouse in individual chambers; recommend production of at least 8 chambers.
- Build a 12” x 8” x 7” AST chamber; walls and start gate made of opaque white acrylic that is easy to clean with 70% ethanol. The guillotine-style, removable starting gate separates chamber into a waiting area (5” x 8”) and testing area (7” x 8”).
- Include a Clear 5”x7” removable partition that separates two sides of testing area evenly; cut three ¼” diameter holes into bottom portion of partition to allow sniffing access, spaced 1” apart and 1” from the front of the partition (Figure 1C).
2. Procedure Overview (Figure 2)
- Prior to AST, house mice individually and allow them to adjust to a reverse light cycle for at least one week.
- Acclimate, train and test each animal in individual testing chambers. Assign each chamber to one mouse and clean with 70% ethanol after finishing the final day of testing. This will minimize stress during testing by making the chamber smell like “home-cage” or “self”.
- Scent the testing pots 2 days prior to testing to allow the scent to dissipate; mice will not dig in pots that have a very strong odor. Using a syringe with a 25 G needle, add approximately 0.1 ml of essential oil to the top of the filter paper.
3. Handling (Days 1-8)
- Handle mice starting 8 days prior to the first day of food restriction. This will reduce stress associated with handling during testing.
- Weigh and record body weights each day of handling. Handle each mouse 2-3 min per day.
- If multiple experimenters are to be involved in testing a set of mice, ensure that each experimenter participates in the handling process to increase familiarity with experimenters and reduce stress and anxiety experienced on training or testing days.
- Continue handling and recording weights throughout the duration of testing.
4. Food restriction (Days 9-12)
- Beginning 4 days prior to the start of acclimation, place mice on a restricted diet to maintain mice at 80-85% of their free feeding body weight. Give 1 gram of food per mouse per day. If body weight falls below the specified range give 2 grams per day until they return to the appropriate range.
- Place 2 ramekins at the front of each home cage in order to allow mice to acclimate to the testing pots. These are the same spots used to train the mouse to dig for a food reward.
- Add 2 grams of food and 1 piece of food reward (half a piece of cereal in each) in the ramekins each day for 4 days.
- If behavior is to be performed in a separate room than the one in which the mice are housed, beginning on day 1 of food restriction, transport mice to the testing room for at least 1 hr per day prior to returning to the housing room to allow them to acclimate to the testing room.
- On the last day of food restriction, before the start of acclimation, change the home cage bedding. The cage bedding will not be changed again until after testing is complete to reduce stress or anxiety experienced during training and testing (unless absolutely necessary).
- Maintain mice on food restriction until the end of testing. On days 13-17, give mice food after acclimation, training, or testing based on an estimate of the amount of food reward consumed (generally <1g of food pellet).
5. Acclimation (Days 13-14)
- Beginning 2 days prior to the start of training, perform acclimation during the dark cycle under red light.
- Spread a small amount of dirty bedding from the home cage in the chamber, this will reduce the stress of being in a new environment by introducing a familiar “home-cage” or “self” smell to the testing chamber (the same chamber will be used for a single mouse for the duration of training and testing).
- Place a clean ramekin with water in the waiting area of the chamber; mice tend not to eat if they do not have access to water.
- Place the two ramekins that have been used for food restriction in the testing area with a food reward (~20 mg cereal piece) in each.
- Place the mouse in the waiting area; remove the start gate and allow the mouse to explore the chamber for 1 hr.
- Continuously add food rewards (~20 mg cereal piece) to the empty ramekins in order to encourage the mice to explore the testing area and the pots frequently.
- Ensure that the mouse can see the experimenter throughout the acclimation period so that the presence of the experimenter is not an added stressor during testing.
6. Training (Day 15)
- Place the mouse in the waiting area.
- Place the empty ramekins, each containing a food reward (~20 mg cereal piece), into the testing area and lift the start gate. Allow mouse 3 min to retrieve both food rewards. Repeat this step several times.
- Gradually add sawdust/mouse cage bedding to the pots in subsequent trials. Allow the mouse 3 min to retrieve the food reward from each pot before ending the trial. Once the mouse has retrieved the food reward from each pot, proceed to the next trial. If the mouse does not make attempts at digging once the food reward is partially covered, hints may be provided (i.e., sprinkle cereal dust over area of the food reward, make an indention in the sawdust over area of food reward, uncover part of the food reward).
- Continue adding sawdust/bedding after each trial until the food reward is fully covered and the mouse reliably demonstrates the ability to dig in a full pot to find the food reward.
- If a mouse has not reliably demonstrated the ability to find a food reward in a full pot within 2 hr of the start of training, continue to a second day of training. Maintain the mouse on food restriction and repeat this procedure the next day.
- If the mouse fails to dig for a food reward after two successive days, exclude it from the remainder of the experiment, and annotate as a “failed to dig” in the experimental record.
7. Testing (Days 16-17):
- On day one, test simple discrimination (SD), compound discrimination (CD), reversal (R1), and intra-dimensional shift (IDS).
- On day two, test the intra-dimensional shift 2 (IDS2), intra-dimensional shift 3 (IDS3), reversal 2 (R2; optional), extra-dimensional shift (EDS).
- Pair media and scents as shown in Table 1.
- At the time of handling on testing day, give mice one food reward to avoid a lack of focus during testing due to hunger. From this point until the end of the testing day, do not provide any food except rewards for correct choices.
- At the start of testing, using forceps (limits the spread of scents between pots), place a piece of the food reward in the pot that indicates a correct choice. The food reward should be fully covered by media (see Figure 3). To avoid a scent cue from the food reward, sprinkle cereal dust over all pots at the start of the stage.
- Place the mouse in the waiting area with the start gate closed. Place the pots on either side of the testing chamber. The trial begins when the start gate is removed and the timer is started. Allow each mouse 3 min per trial to make a choice. Record the choice (correct or incorrect) and time until the mouse made a choice for each trial.
NOTE: A “choice” is indicated by an attempt to dig in either pot. A dig is only recorded as a choice if the mouse has displaced the media and poked its nose far enough in the space it creates to find the food reward or displaced enough of the media to uncover the food reward. If a mouse has not made a choice within 3 min, this is recorded as no choice (incorrect). - Once a choice has been made for either pot, remove the pot that was not chosen from the testing chamber. If a correct choice was made, allow the mouse to finish the food reward before placing the mouse back in the waiting area. If an incorrect choice is made, allow the mouse to explore the pot to show that there is no food reward for that choice before returning the mouse to the waiting area.
- At the end of each trial, place the mouse back in the waiting area and replace the start gate.
- Ensure that the mouse meets the criterion of 8 correct trials consecutively to advance to the next stage of AST. If a mouse does not obtain 8 correct choices consecutively within 50 trials, it fails that stage and cannot move on to complete the test. 6 consecutive no choices (3 min without a choice) is a failure to participate and prohibits the mouse from moving on to the next stage.
Representative Results
The typical dependent measure in this test is the number of trials per stage to meet criterion (or 8 correct consecutive choices). Figure 4A shows the average number of trials to meet criterion at each stage in untreated C57BL/6J using the AST. As mice form an attentional set on the relevant cue dimension (odor or media), performance will improve, as indicated by a reduction in trials to meet criterion in successive intradimensional shifts, and the reversal stages and the extradimensional shift will require additional trials to meet criterion. It is important to counterbalance the number of animals that start on each of the two relevant dimensions (i.e., media or odor) as we have observed some difference at the simple discrimination stage reflected in an increased number of trials to criterion if odor is the relevant cue (Figure 4B). If the trials to reach criterion at the SD stage are significantly different between the two relevant cue dimensions (i.e., better performance when media is relevant as in Figure 4B), performance data for each mouse during subsequent stages can be normalized to its performance during the simple discrimination stage. As illustrated in Figure 4C, this normalization step reduces variability associated with learning the initially relevant cue and enhances visualization of improved performance on the subsequent IDS stages. Although group sizes of ~20 mice will be sufficient to allow analysis of raw data without normalization. Importantly, a significant increase in the trials to meet criterion during the extradimentional shift relative to the previous intradimentional shifts indicates is an important control measure of the test validity (Figure 4D & 4E). Prior to the ED shift stage, mice learn to pay attention to a single relevant cue dimension (odor or media) in order to locate the reward. The switch to a new relevant cue dimension, that was previously irrelevant, should require more trials to reach criterion, resulting in an ID/ED shift. Interestingly, analysis of the ID/ED shift within the raw data revealed a significant increase in the trials to reach criterion between IDS2 and ED (Figure 4D), and trend toward an increase between IDS3 and ED (data not shown, p<0.1) and an increase between the mean trials to reach criterion of IDS2 and IDS3 compared to ED (data not shown). Interestingly, a significant ID/ED shift was apparent between both IDS2 and IDS3 compared to ED when the data were normalized to performance at the SD stage (Figure 4E).
It is expected that approximately 70-80% of control mice will complete the entire task (20-30% drop out rate). Alternative dependent measures include average latency to make a choice as measured at each stage of the task (although we have found this to be highly variable between mice), total number of errors and number of “set-loss” errors at the reversal and extra-dimensional shift stages as defined by Lapiz-Bluhm et al.14 as the number of times an incorrect choice was made following three or more consecutively correct choices. An increased number of “set-loss” errors may indicate a perseverative behavior. Additionally, mice typically complete each stage of testing in less than 30 min (Figure 5), and the duration of time spent in each stage is a function of trials to reach criterion.
In a previous study using a similar paradigm as detailed here, neurotoxic lesions to the OFC or the mPFC in mice were shown to impair performance at the reversal and extra-dimensional shift stages respectively, validating the use of this test to determine functional deficits in the same regions as rat and non-human primate studies11. While the sequence of the tasks in the aforementioned study varies slightly to that shown here, it is expected that lesions or deficits in the OFC and mPFC will also result in impaired performance at R1 and EDS, as reflected by an increase in the number of trials to criterion at those stages. .
| Stage | Relevant | Examples | Relevant | Examples | ||
| Simple Discrimination (SD) | Medium | Felt (+) | Paper (-) | Odor | Nutmeg (+) | Rosemary (-) |
| Compound Discrimination (CD) | Medium | Felt (+)/Nutmeg | Paper (-)/Rosemary | Odor | Nutmeg (+)/Felt | Rosemary (-)/Paper |
| Felt (+)/Rosemary | Paper (-)/Nutmeg | Nutmeg (+)/Paper | Rosemary (-)/Felt | |||
| Reversal 1 (R1) | Medium | Paper (+)/Rosemary | Felt (-)/Nutmeg | Odor | Rosemary (+)/Paper | Nutmeg(-)/Felt |
| Paper (+)/Nutmeg | Felt (-)/Rosemary | Rosemary(+)/Felt | Nutmeg (-)/Paper | |||
| Intradimensional Shift (IDS) | Medium | Pompoms | Sequins (-)/Clove | Odor | Cinnamon (+)/Pompoms | Clove (-)/Sequins |
| (+)/Cinnamon | ||||||
| Pompoms (+)/Clove | Sequins (-)/Cinnamon | Cinnamon (+)/Sequins | Clove (-)/Pompoms | |||
| IDS2 | Medium | Pipecleaners | Googly eyes (-)/Ginger | Odor | Red thyme (+)/Pipecleaners | Ginger (-)/Googly eyes |
| (+)/Red thyme | ||||||
| Pipecleaners (+)/Ginger | Googly eyes (-)/Red thyme | Red thyme (+)/Googly eyes | Ginger (-)/ Pipecleaners | |||
| IDS3 | Medium | Ribbon (+)/ Vanilla | Metallic strips (-) /Lemon | Odor | Vanilla (+)/Ribbon | Lemon (-)/Metallic strips |
| Ribbon (+)/Lemon | Metallic strips (-) /Vanilla | Vanilla (+)/Metallic strips | Lemon (-)/Ribbon | |||
| R2 | Medium | Metallic strips (+)/Vanilla | Ribbon (-)/Lemon | Odor | Lemon (+)/Ribbon | Vanilla (-)/Metallic Strips |
| Metallic strips (+)/Lemon | Ribbon (-)/Vanilla | Lemon (+)/Metallic strips | Vanilla (-)/Ribbon | |||
| Extradimensional Shift (EDS) | Odor | Citronella (+)/Raffia | Anise (-)/Foam | Medium | Raffia (+)/Citronella | Foam (-)/Anise |
| Citronella (+)/Foam | Anise (-)/Raffia | Raffia (+)/Anise | Foam (-)/Citronella | |||
Table 1. Example combinations of odor and digging medium. The scents and digging media in these combinations have been validated in this study. Representative results obtained using these specific combinations are shown in Figure 2.
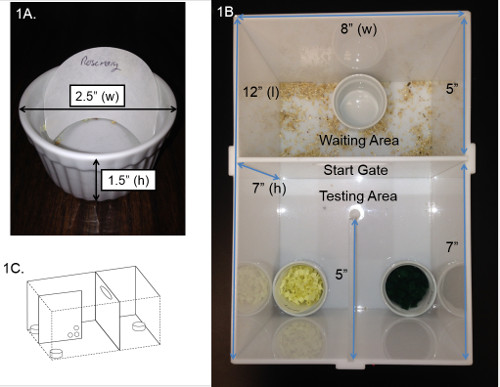
Figure 1. AST testing chamber. (A) Picture and dimensions of ramekin and example of filter paper placement. (B) Picture of the AST testing chamber adapted for mice and (C) a three dimensional diagram of the testing chamber illustrating placement of holes within the partition.
Figure 2. Digging medium. Examples of digging medium pairs used in this protocol as detailed in the materials list (ii.-xi.). Pots are filled approximately to the inner rim with digging medium.

Figure 3. Timeline for testing.
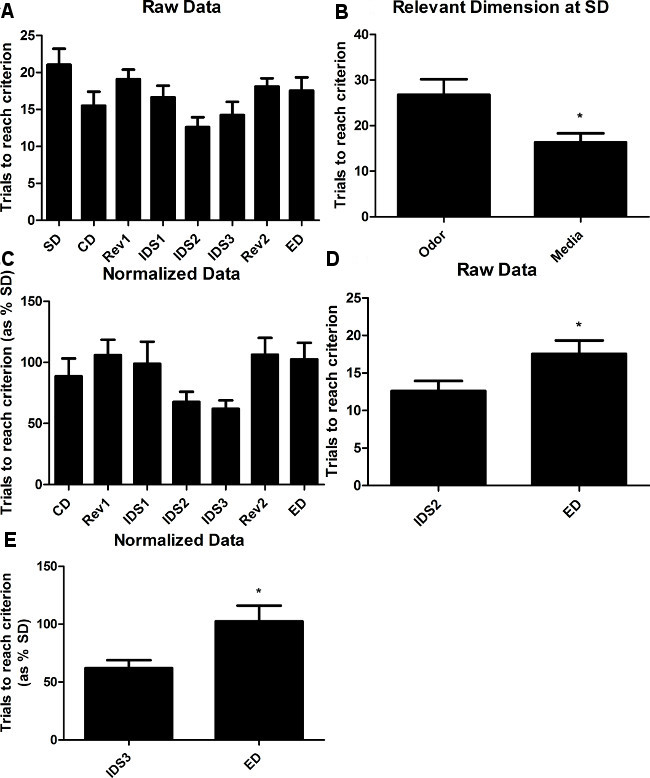
Figure 4. C57Bl/6J performance in the AST. (A) Average number of trials to meet criterion in the AST for male C57BL/6J mice. Data represent mean ± SEM (n=22 mice/group), p=0.013 for main effect of stage. (B) Average number of trials to reach criterion at the SD stage for distinct initial relevant cue dimensions. Data represent mean ± SEM (n=22 mice/group), *p=0.01. (C) Normalized data represented as a percentage of each mouse’s individual performance at the SD stage. Data represent mean ± SEM (n=22 mice/group), p=0.06 for main effect of stage. (D) ID/ED shift in trials to reach criterion between IDS2 and ED. Data represent mean ± SEM (n=22 mice/group), *p=0.03. (E) ID/ED shift in normalized data between IDS3 and ED. Data represent mean ± SEM (n=22 mice/group), *p=0.012.
Discussion
Deficits in cognitive flexibility mediated by the PFC represent a significant disability associated with a variety of neurological and neuropsychiatric disorders, and can have a major impact on outcomes and quality of life of individuals suffering from these disorders. Optimization of the AST for use in mice is vital to investigating the genetic underpinnings in diseases coupled to prefrontal cortical mediated cognitive dysfunction. We have found that the method described above is a reliable measure of a specific cognitive deficit, cognitive flexibility, and closely approximates the results obtained using this task in rats and non-human primates. The method described is the result of an extensive survey of previously described protocols in rats and mice as well as advice and guidance of experts at our institution.
Previous reports using similar tasks in mice have demonstrated the adaptability of such a behavioral measure, albeit with several caveats2,6,11. Colacicco et al. limited the number of discriminations tested per day to 2, which spread testing over a four day period11. This included repetitions of previously learned stimuli pairs on consecutive testing days. While effective, the aim of developing the protocol presented here is to minimize the duration of the testing period and maximize efficiency. Here, we limit testing to a two day period and eliminate repetitions of previously learned stimuli pairs. Previous researchers have noted that mice require overtraining to form an attentional set and exhibit a small “window of testability” in which they will maintain effortful responding. Our protocol also involves modest overtraining on the intradimensional shift within the initially relevant cue dimension. Particularly when evaluating the normalized data in Figure 4C, it appears that mice tested in our protocol may exhibit a more pronounced deficit during the second intradimensional reversal (relative to the immediately preceding stage). This observation is consistent with the notion of overtraining in order to ensure formation of an attentional set, as mice also exhibited an ID/ED shift. One possibility as to why mice sustain effortful performance in our protocol (Figure 5), which involves an average session length of 2 hr, may be due to utilization of a reverse light cycle. We tested behavior in an isolated and dedicated testing room under red lighting conditions during the first four hours of the dark (wakeful) cycle. However, systematic testing outside the scope of this method manuscript would be required to determine what factors contribute to the duration in which mice will continue to perform the AST, and if deficits in olfactory discrimination are suspected, prescreening may be warranted prior to testing as alternate odor-free methodologies have been reported.
Additionally, during the initial method development, we discovered that young adult mice (8-12 week old) performed with greater variability that slightly older 4-6 month old mice. We speculate that the food restriction protocol affects growing mice differently than more mature mice, and while, in a classic neurodevelopment sense, the mouse brain is considered fully developed by 6 weeks, it is possible that the transition from adolescent to adult maturity and early adulthood synaptic remodeling within the prefrontal cortex confound AST performance in younger mice. However, these fascinating questions remain to be explored.
Other variations of AST protocols in mice include “free” trials, meaning a designated number of trials (typically 4) at the beginning of each stage are not scored, and the animal is allowed to explore both pots until the reward is retrieved. The protocol detailed here does not include any “free” trials. In addition, we set the requirements to meet criterion more stringent (8 consecutively correct trials to criterion instead of the previously described 8/10 trials to meet criterion) and have found that this is an effective indicator of whether the animal has truly learned the rule. Garner et al. showed that a period of overtraining following an IDS (i.e., presentation of the previously relevant stimuli for an additional 50 trials) significantly increased the trials to criterion in the EDS2. Here, we include multiple intra-dimensional shifts, in an effort to “overtrain” and ensure the formation of an attentional set on the relevant dimension, in agreement with Bissonette et al. 6. Additionally, Garner et al. limited testing sessions to approximately 1 hr (or when the mouse no longer responded)2, whereas we have found that C57BL6/J mice adequately and reliably progress through all four stages presented to them each day within approximately a 2 hr period (for control mice). Although, potential differences in motor ability between experimental groups should be considered when interpreting performance. Our procedure restricts the time and labor investment to 17 days for each animal from the start of handling through the completion of testing. It is recommended that an individual experimenter limit the number of mice tested in a single day to no more than 2 (this can be modified based on individual experimenter ability and experience), which allows a single experimenter the ability to complete testing on at least 6 mice per week. If motor function is mildly impaired, the length of each trial can be increased, as correct responding, not speed of responding, is the key dependent variable.
Visual discrimination paradigms similar to those that have been used to assess cognitive performance in non-human primates have recently been adapted for use in rodents, and can be used as an alternative to the odor and medium discrimination tasks described here. This approach may be necessary if significant differences in olfactory discrimination are present between experimental groups of mice. Brigman et al. have shown that mice readily discern compound visual stimuli in an ID and ED shift paradigm15. While this automated method better represents the test employed to study prefrontal cortex dysfunction in humans and non-human primates, the cost of the operant chambers and touchscreens required to perform this automated test in mice can be prohibitive.
It is recommended that behavior be performed as consistently as possible for each mouse, i.e., habituation, training and testing should be performed at the same time of day for each animal. Furthermore, the behavior room should remain unchanged throughout the experiment, making the use of shared/community space problematic due to uncontrollable traffic flow, odor introduction, etc. If animals are to be transported from a housing room to a remote behavior room (as is the case here), the same route should be taken each time. The behavior room should be in an area where the experimenter and the animals will not be disturbed during the testing procedure. If multiple experimenters are performing the task in the same room, entry and exit from the room should be as limited as possible to avoid any loud noises or disruption which may increase the stress or anxiety of the animal during testing. Additionally, experimenters should be careful in removing and replacing the start gate as well as the pots into and out of the chamber so as to create as little noise and disruption as possible. Experiments performed in rats have demonstrated that AST performance can be disrupted by various stressors.
Scenting of the pots should be performed each week and on the same day each week, at least 2 days prior to the first testing day. It is recommended that the pots are scented in a well-ventilated area outside of the area where the test is to be performed (e.g separate laboratory, hallway, fume hood). This will help to avoid cross contamination of the scents and will avoid confusing the mice with scents that are not part of the test. Additionally, prior to and during testing, strong scents which may distract the animal from the discrimination (i.e., consumption of gum or coffee immediately prior to testing, strong perfume/deodorant, etc.) should be avoided for the aforementioned reason. Should experimenters desire to use alternate digging medium than what is described in this protocol, it is recommended that the medium be of similar density and weight to that described here (excessive weight/density makes it difficult for the mouse to dig adequately). For example, we found that small plastic beads were not suitable because they were difficult for the mice to push aside. Additionally, any media with a very strong scent (e.g., rubber strips and some plastic material) is not suitable as it may interfere with the ability to detect the scents being tested.
Training and testing of an individual mouse should be performed by a single experimenter, however acclimation can be done by any experimenter as long as they have participated in handling. All pots to be used in the four stages performed each day should be within reach so as to limit the amount of time the animal is in the waiting area between stages. This will help maintain the attention of the mouse on each task. Furthermore, once testing has begun it is important not to stop until the completion of the tasks for that day of testing (i.e., all 4 stages for each testing day must be completed in succession).
In conclusion, the protocol described here provides detailed methodological instructions for effective utilization of the AST to measure prefrontal cortical-dependent cognitive function in mice, including description of data analysis and potential caveats. A variety of mouse AST methods are reported in the primary literature, each with its own distinct procedural detail. The unique coupling of both text-based and visual information will be particularly helpful for researchers attempting to incorporate this powerful behavioral assay into their scientific repertoire.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by grants R01-MH090127, P30-MH089868 from the National Institute of Mental Health and award number UL1TR001120 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or NCATS. Additionally, we would like to gratefully acknowledge Dr. David Morilak for his valuable expertise in guiding the early method development.
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description | |
| Testing Chamber | Fabricated in-house, specifications can be found in protocol | |||
| 3 0z. Ramekins | BIA Cordon Bleu | 900002S12 | Purchased on amazon.com | |
| Essential Oils: | Fronteir Natural Products Co-Op | Purchased on http://www.frontiercoop.com/prodlist.php?ct=anpceoeo | ||
| Clove Bud | 191114 | |||
| Red Thyme | 191140 | |||
| Lemon | 190810 | |||
| Cinnamon Leaf | 191111 | |||
| Rosemary | 191133 | |||
| Citronella Java | 191112 | |||
| Vanilla in jojoba oil | 191231 | |||
| Anise Seed | 191102 | |||
| Ginger | 191121 | |||
| Nutmeg | 191161 | |||
| Filter Paper, 9.0 cm diameter | VWR International | 28310-048 | Purchased on https://us.vwr.com/ | |
| Digging Media | ||||
| Raffia | Ashland 8 oz Raffia | n/a | Purchased on http://www.michaels.com/ Cut into 1/2" to 1" strips |
|
| Green Felt | Creatology Basic Felt Forest/Dark Green 36 in x 36 in | n/a | Purchased on http://www.michaels.com/ cut into 1 cm x 1 cm squares |
|
| Brown foam | Creatology Fun Foam 18 x 12 in sheets (light brown/tan) | n/a | Purchased on http://www.michaels.com/ cut into 1/2 cm x 2 cm rectangles |
|
| Crepe paper (yellow) | Celebrate It Paper Crinkle 4 oz yellow jumbo | n/a | Purchased on http://www.michaels.com/ Cut into 1/2" to 1" strips |
|
| Ribbon (turqoise) | Celebrate It Wide Ribbon 4 in x 10 yd 100% polyester | n/a | Purchased on http://www.michaels.com/ cut into 1 cm x 1 cm squares |
|
| Metallic | Celebrate It Metallic Crinkle Gold 2 oz | n/a | Purchased on http://www.michaels.com/ Cut into 1/2" to 1" strips |
|
| Googly Eyes, 4 mm | ME4144 | Purchased on http://factorydirectcraft.com/index.php | ||
| Black Sequins, 3 mm | 8780BK | |||
| Pink Pom Poms, 1/4 inch | 1018221 | |||
| Pipe Cleaners, red | 108430 | |||
| Bedding material/sawdust | Obtained from in-house animal resources | |||
| Timer | Control Company | 5000 | Purchased on http://www.control3.com/5000p.htm | |
| Lightly sweetened dry breakfast cereal loops | HEB | Honey nut toasted oats used in the protocol described, any lightly sweet coated breakfast cereal is recommended | ||
References
- Birrell, J. M., Brown, V. J. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 20 (11), 4320-4324 (2000).
- Garner, J. P., Thogerson, C. M., Wurbel, H., Murray, J. D., Mench, J. A. Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behav Brain Res. 173 (1), 53-61 (2006).
- Nagahara, A. H., Bernot, T., Tuszynski, M. H. Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol Aging. 31 (6), 1020-1031 (2010).
- Rock, P. L., Roiser, J. P., Riedel, W. J., Blackwell, S. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. , 1-12 (2013).
- Stuss, D. T., et al. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 38 (4), 388-402 (2000).
- Bissonette, G. B., et al. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 28 (44), 11124-11130 (2008).
- Chase, E. A., Tait, D. S., Brown, V. J. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. Eur J Neurosci. 36 (3), 2368-2375 (2012).
- McAlonan, K., Brown, V. J. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 146 (1-2), 97-103 (2003).
- Dias, R., Robbins, T. W., Roberts, A. C. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 110 (5), 872-886 (1996).
- Bissonette, G. B., Powell, E. M. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 62 (3), 1168-1174 (2012).
- Colacicco, G., Welzl, H., Lipp, H. P., Wurbel, H. Attentional set-shifting in mice: modification of a rat paradigm, and evidence for strain-dependent variation. Behav Brain Res. 132 (1), 95-102 (2002).
- Kos, T., Nikiforuk, A., Rafa, D., Popik, P. The effects of NMDA receptor antagonists on attentional set-shifting task performance in mice. Psychopharmacology (Berl). 214 (4), 911-921 (2011).
- Tait, D. S., Chase, E. A., Brown, V. J. Attentional Set-Shifting in Rodents: a Review of Behavioural Methods and Pharmacological Results. Curr Pharm Des. , (2013).
- Lapiz-Bluhm, M. D., et al. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol. 20 (10), 1115-1137 (2008).
- Brigman, J. L., Bussey, T. J., Saksida, L. M., Rothblat, L. A. Discrimination of multidimensional visual stimuli by mice: intra- and extradimensional shifts. Behav Neurosci. 119 (3), 839-842 (2005).

