Minimally-invasive Technique for Injection into Rat Optic Nerve
Summary
Direct injection into the rat optic nerve is useful for regenerative research. We demonstrate a minimally-invasive technique for direct injection into a rat optic nerve that does not involve opening the skull. Using this method, surgical complications are minimized and recovery is more rapid.
Abstract
The rat optic nerve is a useful model for stem cell regeneration research. Direct injection into the rat optic nerve allows delivery into the central nervous system in a minimally-invasive surgery without bone removal. This technique describes an approach to visualization and direct injection of the optic nerve following minor fascial dissection from the orbital ridge, using a conjunctival traction suture to gently pull the eye down and out. Representative examples of an injected optic nerve show successful injection of dyed beads.
Introduction
The optic nerve provides an ideal location for central nervous system (CNS) regenerative research including ophthalmologic conditions such as optic neuritis, glaucoma and trauma. Injections of a variety of stem cells have either demonstrated efficacy or shown promise in replacing lost myelin, increasing axonal count and/or preventing degenerative diseases.1,2
The human optic nerve contains approximately 1.2 million parallel axons traveling from the retina to the chiasm with a diameter of approximately 3.0-3.5 mm.3 To model human diseases in the laboratory, the rat has been used frequently. The adult rat optic nerve contains approximately 100,000 axons within a diameter of approximately 0.5 mm.4 One of the major limitations in CNS regenerative research is direct boneless access. Complications and surgical risks to the animal are higher when the skull or vertebrae are removed. Similar to the benefits of minimally-invasive approaches in the spine,5 direct optic nerve injections without opening the skull offer reduced complications and a more rapid recovery.
This technique has been used in previous studies.6 In this manuscript and accompanying video, we demonstrate a minimally-invasive procedure to inject stem cells into the rat optic nerve.
Protocol
NOTE: All animal procedures were approved by the Johns Hopkins Animal Care and Use Committee. Anesthesia machines require yearly inspection and calibration as necessary.
1. Anesthesia and Positioning
- Anesthesia.
- Perform all surgical procedures under anesthesia with 2-3% isofluorane. Confirm appropriate level of anesthesia by toe pinch and breathing rate. Check that the rat does not flinch in response to a toe pinch.
NOTE: A flinch indicates anesthesia that is too late and may require longer anesthesia before beginning or a higher isofluorane concentration. A breathing rate less than 1 breath every 2 sec is too slow indicating the anesthesia is too high and may require lightening of the isofluorane concentration. - Secure the animal as necessary to avoid head movement during the procedure. Place a drop of lidocaine on the surgical eye. Use artificial tears every 10 min to prevent dryness while under anesthesia. Give an injection of buprenorphine of 0.01 mg/kg SC pre-operatively and then every 6-8 hr as needed.
- Perform all surgical procedures under anesthesia with 2-3% isofluorane. Confirm appropriate level of anesthesia by toe pinch and breathing rate. Check that the rat does not flinch in response to a toe pinch.
- Positioning.
- Place rats into a stereotaxic frame and keep warm with a heating pad. Wet the scalp fur with alcohol taking care to avoid exposure on the eyes. Use sterile tools and sterile technique to minimize risk of post-operative infections.
2. Eye Control
- Place a 4-0 suture in the lateral conjunctiva and tie it with enough suture to permit gentle traction.
3. Dissection
- Initial Dissection.
- Make a ~1 inch incision in the skin overlying the orbital ridge using a size 10 scalpel as shown in Figure 1. Retract the skin and the underlying fascia and carefully dissect away the fascia. To prevent excessive bleeding into the surgical, avoid cutting blood vessels while dissecting the fascia. Use strategically placed cotton tips to provide hemostasis.
- Deeper Dissection.
NOTE: With gentle traction on the conjunctiva pulling the eye down and out of the socket, the superior orbital muscle will come into view. In order to expose the optic nerve, this muscle must be cut and the retro-orbital fat removed. The fat can be discarded and should not be replaced after injection. From this point, the optic nerve fascia should be visible as a bundle of the optic nerve itself, along with blood vessels wrapped in dura (Figure 1).- Make a small incision in the dura using the scalpel or a 31 gauge beveled needle to make piercing less traumatic.
4. Pipette Injector
- Pull a glass micropipette to a diameter of 50-100 µm. To provide stability, mount the micropipette on a micromanipulator and attach to a Hamilton syringe connected to an infusion pump.
- Pull up the beads (or stem cells) reconstituted in a 0.5-1.0 µl volume into the micropipette retrograde along with 0.5 µl volume of methyl blue solution before and after.
NOTE: An infusion rate set to 0.5-2 µl per min prevents trauma to the optic nerve.
5. Injection
- Lower the tip of the micropipette onto the optic nerve just above the nicked dura.
NOTE: A tiny, but brisk movement of the glass tip into the optic nerve results in the least damage. As the infusion pump begins, the methyl blue dye should highlight the area of the injected optic nerve. The dye should remain localized within the optic nerve without leaking out in the subarachnoid space. - Follow the second band of methyl blue to determine when the stem cell injection is complete and turn the infusion pump off as the second dose of methyl blue is injected. Keep the micropipette still within the optic nerve for 2 min for each 1 µl volume injected into to prevent high pressure ejection upon withdrawal of the micropipette.
NOTE: An alternative approach is to connect the pipette tip to an air filled syringe that can be manipulated manually. Either way, avoid excessive force to minimize damage to the optic nerves. We recommend injecting no more than 2 µl of volume in each optic nerve.
6. Follow up
- Immediate.
- When the injection is complete, remove the micropipette along with any cotton tips used to provide hemostasis. Suture the skin with 3-0 silk and remove the conjunctival suture.
- Keep rats warm on a heating pad until they emerge from anesthesia. Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency. Do not return an animal that has undergone surgery to the company of other animals until fully recovered.
- If the animal is exhibiting signs of pain including excessive lethargy, ataxia, or labored breathing, administer appropriate analgesia with intramuscular buprenorphine (0.05 mg/kg) up to three times daily.
- Long term.
- For the next 24-48 hr, observe the rat for complications of surgery including swelling or discharge from the wound or other signs of pain such as vocalizing, hunched appearance, non-grooming, or not eating. Consider consultation with a veterinarian or ethical euthanasia as necessary.
Representative Results
At the conclusion of the experiment, rats were sacrificed and perfused with 4% paraformaldehyde. The optic nerves were carefully dissected and mounted for cryostat sectioning. Figure 2 shows an example of a rat whole optic nerve at low power in which Evans blue dye was injected in order to visualize the site. The arrow identifies the precise location of the injection. This dissection was done within a few min of the injection as indicated by the restricted diffusion of the dye down the nerve. In other injections, we have observed a slow diffusion of dye towards the optic chiasm over the course of several hours.
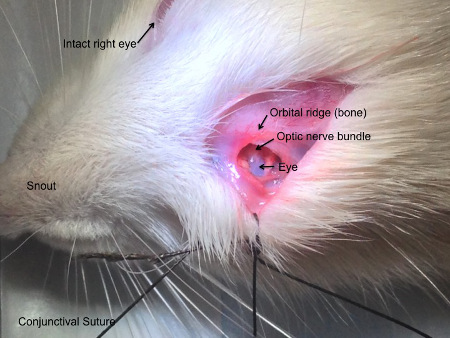
Figure 1: Surgical field of view. The rat is positioned with to access the left eye in this figure. An incision is made above the orbital ridge and the fascial tissue is dissected down behind the eye. A conjunctival suture allows the operator to apply gentle traction which pulls the intracranial optic nerve into view without opening the skull.

Figure 2: Gross dissection of injected optic nerve. Utilizing Evans blue dye, the injection site in the optic nerve can be grossly visualized under a dissection microscope. In this image, the optic nerve was cut at the injection site to show the dye embedded within the optic nerve tissue.
Discussion
Direct injection into the optic nerve of stem cells or other products intended to facilitate regeneration provides a convenient model compared to other means of injections into the CNS. This technique takes less time, requires less total anesthesia, avoids drilling or removing skull or bone tissue, reduces complications rates and allows for more rapid recovery following surgery.
The most critical steps in this protocol include: 1. Adequate hemostasis in the surgical field to allow clear visualization of the optic nerve bundle, 2. Precise measurement of the pipette tip to allow easy insertion into the optic nerve with minimal trauma, 3. Tight control of eye using conjunctival suture which allows optimal exposure of the optic nerve.
An alternative lateral approach to the optic nerve has been described previously which also allows injection directly into the optic nerve without dissection into the skull bone.6 In our experience, the lateral view is more limited to the very proximal optic nerve just behind the retina. In our superior approach, a larger portion of the optic nerve is visible allowing injection into both the proximal and mid-optic nerves.
The major limitation of optic nerve injections is based on the rat’s limited use of vision. Rats can function well with their other senses of touch, hearing and smell and do not necessarily depend on vision as much as humans. Therefore, behavioral studies of rat vision have been limited to instinctual optokinetic reflexes and do not reliably correlate with an intact optic nerve.
However, pathological assessments of axonal count and integrity, and myelination can provide useful information about the benefit of regenerative therapies. Furthermore, the pathological changes in the optic nerve are likely to represent changes in other parts of the CNS such as the brain and spinal cord that are more difficult to access.
The most common current and future application of this procedure is for placement of regenerative stem cells into damage optic nerves. Various disorders of the optic nerve including trauma,7 inflammatory,8 and vascular9 etiologies can potentially benefit from regenerative strategies. Localized injections of small molecules and nanoparticles10 into the optic nerve are also being evaluated for regenerative potential.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by NeuralStem, Inc., and Johns Hopkins Project RESTORE.
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Lewis rat | Charles River | 4 | Any rat strain will work. |
| Anesthesia machine | Surgivet | CDS9000 | CDS 9000 Small Animal Anesthesia Machine – Pole Mount |
| Infusion pump | Stoelting | 53129 | |
| Dissection microscope | National Optical | 409-411-1105 | |
| Fiber-optic light source | Fisher Scientific | 12-562-21 | |
| Dissection and Stereotaxic Instrument | Stoelting | 51400 | |
| Pipette Puller | Kopf | 750 | |
| Pipettes | World Precision Instruments | 18150-6 | |
| Disposable scalpel blades | Harvard Apparatus | 810-15-021 | |
| Iridectomy scissors | Electron Microscopy Sciences | Uniband LA-4XF |
References
- Dahlmann-Noor, A., Vijay, S., Jayaram, H., Limb, A., Khaw, P. T. Current approaches and future prospects for stem cell rescue and regeneration of the retina and optic nerve. Canadian journal of ophthalmology Journal canadien d’ophtalmologie. 45 (4), 333-341 (2010).
- Quigley, H. A., Iglesia, D. S. Stem cells to replace the optic nerve. Eye. 18 (11), 1085-1088 (2004).
- Ghaffarieh, A., Levin, L. A. Optic nerve disease and axon pathophysiology. International review of neurobiology. 105, 1-17 (2012).
- Fukui, Y., Hayasaka, S., Bedi, K. S., Ozaki, H. S., Takeuchi, Y. Quantitative study of the development of the optic nerve in rats reared in the dark during early postnatal life. Journal of anatomy. 174, 37-47 (1991).
- Celestre, P. C., et al. Minimally invasive approaches to the cervical spine. The Orthopedic clinics of North America. 43 (1), 137-147 (2012).
- Hallas, B. H., Wells, M. R. A Novel Technique for Multiple Injections into the Mammalian Optic Nerve. Kopf Carrier. 54, (2001).
- Harvey, A. R., Hellstrom, M., Rodger, J. Gene therapy and transplantation in the retinofugal pathway. Progress in brain research. 175, 151-161 (2009).
- Adachi-Usami, E. Optic neuritis–from diagnosis to optic nerve transplantation. Nippon Ganka Gakkai zasshi. 104 (12), 841-857 (2000).
- Slater, B. J., Vilson, F. L., Guo, Y., Weinreich, D., Hwang, S., Bernstein, S. L. Optic nerve inflammation and demyelination in a rodent model of nonarteritic anterior ischemic optic neuropathy. Investigative ophthalmology & visual science. 54 (13), 7952-7961 (2013).
- Zarbin, M. A., Arlow, T., Ritch, R. Regenerative nanomedicine for vision restoration. Mayo Clinic proceedings. 88 (12), 1480-1490 (2013).

