The Mesenteric Lymph Duct Cannulated Rat Model: Application to the Assessment of Intestinal Lymphatic Drug Transport
Summary
Here we describe a technique to cannulate the mesenteric lymph duct in rats which enables quantification of lipid and drug transport via the lymphatic system following intestinal delivery. The technique can be adapted to assess mesenteric lymph concentrations and/or transport of fluid, immune cells, peptides, proteins and lipophilic molecules.
Abstract
The intestinal lymphatic system plays key roles in fluid transport, lipid absorption and immune function. Lymph flows directly from the small intestine via a series of lymphatic vessels and nodes that converge at the superior mesenteric lymph duct. Cannulation of the mesenteric lymph duct thus enables the collection of mesenteric lymph flowing from the intestine. Mesenteric lymph consists of a cellular fraction of immune cells (99% lymphocytes), aqueous fraction (fluid, peptides and proteins such as cytokines and gut hormones) and lipoprotein fraction (lipids, lipophilic molecules and apo-proteins). The mesenteric lymph duct cannulation model can therefore be used to measure the concentration and rate of transport of a range of factors from the intestine via the lymphatic system. Changes to these factors in response to different challenges (e.g., diets, antigens, drugs) and in disease (e.g., inflammatory bowel disease, HIV, diabetes) can also be determined. An area of expanding interest is the role of lymphatic transport in the absorption of orally administered lipophilic drugs and prodrugs that associate with intestinal lipid absorption pathways. Here we describe, in detail, a mesenteric lymph duct cannulated rat model which enables evaluation of the rate and extent of lipid and drug transport via the lymphatic system for several hours following intestinal delivery. The method is easily adaptable to the measurement of other parameters in lymph. We provide detailed descriptions of the difficulties that may be encountered when establishing this complex surgical method, as well as representative data from failed and successful experiments to provide instruction on how to confirm experimental success and interpret the data obtained.
Introduction
Lymph flows from the small intestine via a unidirectional process that originates at single lacteals that are contained within each small intestinal villi1. Lacteals are relatively permeable to fluid, macromolecules and cells and lymph formation thus commences with the entry of these factors into lacteals. The initial lymph in the lacteals subsequently flows from the intestine via a network of lymphatic microvessels, collecting (afferent) lymphatic vessels, a series of mesenteric lymph nodes and ultimately the post-nodal (efferent) lymph vessels. Within the nodes, lymph passes through a series of medullary sinuses where exchange occurs with node resident immune cells as well as material entering the node from the blood. All lymph flowing from the small intestine eventually converges into the efferent superior mesenteric lymph duct and subsequently the cisterna chyli. The cisterna chyli also collects lymph draining the caudal peripheral tissues, intestinal, hepatic and lumbar regions and joins the thoracic lymph duct together with lymph from the mediastinum and cranial parts of the body. The thoracic lymph duct empties lymph directly into the venous system at the junction of the left internal jugular and subclavian veins. The protocol described here, which enables the collection of lymph directly from the superior mesenteric lymph duct, thus facilitates the analysis of various factors transiting directly from the intestine to the systemic (general) circulation via the intestinal lymphatic system.
The major physiological functions assigned to the intestinal lymphatic system are to maintain fluid balance, to facilitate lipid and lipophilic molecule absorption, and to enable appropriate immune responses1. Tumor cells and viruses also propagate via the intestinal lymphatics2-4 and key changes occur within the lymphatics in several inflammatory and metabolic pathologies5-7. Cannulation of the mesenteric lymph duct to collect lymph within the mesentery enables an analysis of bulk fluid flow via the intestinal lymphatics as well as quantification of the concentration and transport rate of various cells and molecules. Changes to the concentration or transit of these factors in response to various challenges (e.g., diets, antigens, drugs) and in disease models (e.g., colitis, HIV, diabetes) can also be assessed. Whilst it is impossible to extensively describe each lymph component which may be analysed and compared here, mesenteric lymph simplistically consists of aqueous, lipid and cellular phases. Components of interest in the aqueous phase include peptides and proteins such as antigens or tolerogens8, immune messengers such as cytokines and mast cell mediators9, and metabolic mediators such as incretins10. The cellular fraction of the post-nodal mesenteric lymph consists almost entirely (over 99%) of lymphocytes11. Various immune cells (dendritic cells, mast cells, etc.) enter the pre-nodal mesenteric lymphatics but remain within the node12. If the cells within afferent lymph are of interest, it is possible to collect these cells via removal of the mesenteric lymph nodes several days before cannulation of the mesenteric lymph duct12. In this way the afferent and efferent lymph ducts are directly connected and the lymph cells in afferent lymph pass directly into the mesenteric lymph duct. The transit and phenotype of various immune cells passing through the intestinal lymphatics can thus be examined. Perhaps the most common reason cited for collecting mesenteric lymph to date, however, is to study the intestinal processing, absorption and transport of dietary lipids and lipophilic molecules10.
Following ingestion, dietary lipids are digested (for example, from triglyceride to fatty acids and monoglyceride, phospholipid to fatty acids and lysophospholipid, and cholesterol ester to fatty acid and cholesterol, etc.) and dispersed within the intestinal lumen into small micelle and vesicular structures via the addition of amphiphiles from bile (phospholipids, cholesterol and bile salts) and the action of pancreatic enzymes10,13. From here they are absorbed into enterocytes. A proportion of the absorbed components are re-esterified to form triglycerides, phospholipids and cholesterol esters within the absorptive cells (enterocytes). These re-esterified lipids are assembled from a combination of exogenously ingested lipid components and endogenous lipid components from the secreted bile, mucosal lipid pools or the intestinal blood supply13. From here the esterified lipids are either stored within enterocytes or assembled into intestinal lipoproteins (chylomicrons, very low density lipoproteins (VLDL)) together with various apoproteins and other lipophilic molecules (e.g., vitamins)10,13. After exiting enterocytes, lipoproteins are specifically transported from the intestine to the systemic circulation via the mesenteric lymphatic system as the intestinal lacteals are more permeable to their entry than the intestinal blood capillaries. A proportion of absorbed lipid components are also transported from the intestine to the systemic circulation via the blood capillaries and portal vein as single, non-lipoprotein associated, molecules14 . In general, however, the portal vein transport route is only a significant player in the absorption of short and medium chain length lipids.
The collection of mesenteric lymph thus enables the assessment of the transport of lipoproteins and associated components (lipids, lipophilic molecules, apo-proteins) from the intestine. The lipoproteins can be quantitated and characterized with the advantage that mesenteric lymph lipoproteins, in general, are in a nascent state since they have not been extensively modified by systemic enzymes such as lipoprotein lipase15. Whilst the mesenteric lymph cannulated rat model has perhaps historically been most extensively described for the analysis of lipid/lipoprotein transport from the intestine, an area of expanding interest is the role of lymphatics in the transport of lipophilic drugs, prodrugs and other xenobiotics13,16 which is the focus of the model described here. Lipophilic drugs (generally those with log P > 5 and solubility in long-chain triglyceride > 50 mg/g although exceptions are apparent)17,18, prodrugs19 and other xenobiotics13,16 can gain access to the intestinal lymphatics either passively or by actively integrating into intestinal lipoprotein transport pathways19.
The rat mesenteric lymph cannulation technique thus has many applications. Bollman et al. first described a technique to cannulate the mesenteric lymph duct in rats in 194820. Since then a number of variations on the model have been described. For example, collection can occur when the rat is anaesthetized with various anesthetics21,22, or in the conscious state whilst restrained15 or freely moving23,24. Rats can be administered different rehydration solutions and other substances such as lipids and drug formulations at different rates into the stomach, intestine or parenterally (typically 0 – 5 ml/hr)25. In some studies the thoracic lymph duct rather than mesenteric lymph duct is cannulated to estimate transport from the intestine via the lymphatics although this may overestimate transit from the small intestine, depending on the factor of interest, as the thoracic lymph duct also receives lymph from other regions22,26. Lymph cannulation models have also been described in several other species including mice15,27, mini-pigs12, sheep28,29, pigs30 and dogs31. However, the rat model is the most widely and consistently cited. Detailed protocols for cannulation of the mesenteric lymph duct followed by collection of lymph in conscious25 or anaesthetized22 rats and mice15,27 have been published previously and the interested reader is directed to these protocols. This protocol is the first to demonstrate the technique in a visualized format.
The lymph cannulated rat model has advantages over larger animal models in terms of expense, the ease of the surgery and ethical considerations. When compared to the mouse model, mesenteric lymph cannulation surgery is also easier in the rat although the mouse model enables more detailed studies in transgenic animals27. Nonetheless, there are some limitations of the rat model, particularly those associated with differences in physiology, that limit extrapolation to other pre-clinical and clinical situations. For example, in the rat bile flow is constant and independent of food intake whereas in higher species food or lipids stimulate bile flow32. This creates challenges to obtaining representative pre- and post-prandial environments in the rat that reflect what is seen in larger species and humans. For drug delivery studies, larger species may also be preferred when assessing lymphatic transport after the administration of realistic human dosage forms25. In a recent study, lipid transport rates in the mesenteric lymph were found to be comparable across species (mouse, rat, dog) after administration of an equivalent mass and type of lipid which provides some confidence in extrapolating lipid transport data across species27. However, the transport of a model lipophilic drug, halofantrine, ranked in the order of animal size (i.e., dog > rat > mouse). A scaling factor may thus be required to extrapolate lymphatic drug transport data from rat to other species.
A limitation of lymph cannulation models, in general, is that passive lymph collection directly from a lymphatic duct may modify lymph flow and transport since lymph vessels work against a pressure gradient that is altered once the vessel is cannulated33. The lymph cannulation model can also be difficult to establish in laboratories that are unfamiliar with the technique. Alternate models have thus been described. For instance, the transit of factors via the intestinal lymphatic system, such as lipoproteins and lipophilic molecules, has been indirectly studied via collection of blood. One such model involves comparing blood concentrations of lipids and/or drugs following oral administration in the presence and absence of inhibitors (e.g., colchicine, Pluronic L81, cycloheximide) of intestinal lipoprotein production that block lymphatic transport34. An advantage of models that quantify lymphatic transport indirectly via collection of blood samples is that it enables some evaluation of lymphatic transport in humans as invasive surgery is not required35. However, inhibitors of lymphatic transport are not specific and factors that are transported via the lymphatics are diluted and modified in the systemic circulation which complicates such assessments. In vitro alternatives have also been described. For example, caco-2 cell or isolated enterocyte cultures have been utilized to study in more detail the intestinal secretion of molecules that enter the lymphatics36-38. An advanced in vitro model that is more representative of the human intestinal microenvironment was also recently described39. In this model a lymphatic endothelial cell layer is co-cultured with Caco-2 cells which enables more detailed analysis of the transfer of substances from the intestine into the lymphatics. However, in vitro cell systems lack exchange flow and transfer ie interconnection with an intestinal lumen and underlying blood and lymphatic vascular supply. In an alternate approach, Kassis et al. established a dual-channel (high-speed bright-field video and fluorescence) in situ imaging system which enables quantitative comparisons between vessel contraction, lymph flow and fluorescent lipid concentrations in mesenteric lymphatic vessels33. An advantage of this model over the aforementioned in vitro systems is that it enables accurate tracking of the passage of immune cells through the lymphatics. Absolute measurements of mass lipid (or drug) transport are, however, not yet established using imaging methods. In vitro and in silico approaches to specifically predict the extent of lipophilic drug transport via the intestinal lymphatics have also been published40-42. For example, the ex vivo affinity of several compounds for plasma chylomicrons was found to correlate reasonably well with their lymphatic transport in vivo41. Subsequently, the same group established an in silico model to predict drug affinity for chylomicrons based on multiple physicochemical properties40. Holm et al. also established a relatively complex in silico model to outright predict lymphatic transport of lipophilic compounds on the basis of molecular descriptors42. These models may provide a useful approach to predict the extent of lymphatic transport of unknown drugs. Validation of the models with a wide range of drugs and across different labs will, however, be required to confirm their accuracy and reproducibility.
Cannulation of the mesenteric lymph duct thus remains the only means to directly examine the content of lymph draining the small intestine and the transit rate of the complex array of factors (cells, proteins, peptides, lipids, drugs) in lymph in an in vivo situation. Herein we describe a protocol for cannulation of the mesenteric lymph duct and carotid artery that enables the collection of mesenteric lymph and systemic blood from anaesthetized rats. Representative data demonstrate how the model can be used to examine lipid and drug transport from the intestine via the mesenteric lymphatic system. This is followed by a discussion of difficulties that can be encountered in establishing the model and a troubleshooting guide. Once established the model is a powerful tool to investigate intestinal lymphatic transport.
Protocol
The studies described in this manuscript were approved by the local animal ethics committee and were conducted in accordance with the Australian and New Zealand Council for the Care of Animals in Research and Teaching guidelines. Prior to beginning any animal procedure, ensure that the appropriate permission is obtained through the local institution/organization. As with all animal surgeries, ensure that the surgery is performed by appropriately trained operators, under aseptic conditions and that anesthetics, analgesics and antibiotics are administered when required to ensure an ethical and successful outcome.
1. Preparations the Day Before the Surgical Procedure
- Fast the rat the night before surgery if required. Ensure the rat has free access to water.
- Prepare the solutions to be administered to the rat.
- Prepare a rehydration solution such as sterile saline or Ringers solution.
- Prepare anesthetic solutions as required. The anesthetic used in the experiments described here consisted of “Cocktail 1” prepared by combining 1.9 ml of ketamine 100 mg/ml, 0.5 ml of Xylazine 100 mg/ml, 0.2 ml of acepromazine 10 mg/ml and 2.5 ml of saline and “Cocktail 2” consisting of 1 ml of ketamine 100 mg/ml and 0.1 ml acepromazine 10 mg/ml. NOTE: Inhaled isoflurane or sevoflurane may be preferred as it can provide a surgical plane of anaesthesia for a longer period of time.
- Optionally prepare a formulation containing the drug in, for example, a lipid emulsion. Perform appropriate stability studies to assure the stability of the formulation over the period of storage and administration.
NOTE: The exact nature of the formulation and drug to be administered will vary with each study as the aim of lymphatic drug transport studies is most often to evaluate the efficiency of lymphatic drug transport as a function of the formulation and drug administered.- The formulation utilized in the representative experiments in Figure 4 and 5 here is prepared, as described previously43. Briefly, incorporate 14C oleic acid (2-5 µCi) and halofantrine (200 µg) into 40 mg oleic acid prior to dispersion in 5.6 ml of an aqueous phase consisting of 5 mM sodium taurocholate in phosphate buffered saline (pH 6.9).
- For formulations containing 2-monoolein, add 7.1 mg of 2-monoolein along with other components, to the aqueous phase at the same time as the oleic acid phase containing halofantrine. Prepare the formulations containing 2-monoolein immediately prior to administration to the rat as 2-monoolein is relatively unstable and isomerises to 1-monoolein.
- Subsequently emulsify the formulations by ultrasonication for 2 min at RT.
NOTE: As has been described previously43, monitor the stability of the formulations by i) visual inspection of the emulsion under polarized light to ensure that the emulsion is not phase separated, ii) particle size analysis immediately after preparation and following dosing using dynamic light scattering to provide an indication of any phase changes and/or separation, and iii) analysis of halofantrine concentrations using a validated HPLC assay.
- Prepare anti-coagulant solution containing 10 mg/ml EDTA in sterile water or 10 IU/ml heparin in saline to flush the lymph cannula. Also prepare anti-coagulant solution containing 2.5 – 10 IU/ml heparin in saline to flush the carotid artery cannula.
- Prepare the polyethylene cannulas for insertion into the mesenteric lymph duct (0.8 mm O.D, 0.5 mm I.D.), carotid artery (0.96 mm O.D, 0.58 mm I.D. or 0.8 mm O.D, 0.5 mm I.D.) and duodenum (0.96 mm O.D, 0.58 mm I.D.)
- Cut the cannulas to the required length (typically 25 – 30 cm for the carotid artery and duodenal cannula and 10 – 15 cm for the lymph cannula) and place a bevel at the tip of the cannulas using a sterile surgical blade (see Figure 1).
NOTE: This increases the ease of cannula insertion and reduces the incidence of cannula blockage post-insertion. - Place a J shape anchor point in the intraduodenal cannula by looping a small portion of the cannula back on itself, heating it with a lighter or hot plate and then cutting to size (see Figure 1).
- Cut the cannulas to the required length (typically 25 – 30 cm for the carotid artery and duodenal cannula and 10 – 15 cm for the lymph cannula) and place a bevel at the tip of the cannulas using a sterile surgical blade (see Figure 1).
- Attach the lymph cannula to a 25 G needle and syringe containing an anti-coagulant solution (prepared in step 1.3). Fill the lymph cannula with the anti-coagulant solution and leave the solution in the cannula O/N to reduce the incidence of clot formation in the lymph cannula during lymph collection.
- Prepare tubes for lymph and blood sample collection. Pre-weigh lymph collection tubes, label tubes for lymph and blood sample collection and add anti-coagulant solution. Add sufficient anti-coagulant solution to achieve a final concentration of 1 mg/ml EDTA in sterile water or 10 – 20 IU/ml heparin in the collected lymph or blood.
2. Preparations Immediately Prior to Commencing the Surgical Procedure
- Check all cannulas by flushing with sterile saline or anti-coagulant solution to ensure that they are patent.
- Prepare the rat for the surgical procedure.
NOTE: The mesenteric lymph duct is more visible in rats that have eaten or been administered a dose of oil as the mesenteric lymph becomes milky and opaque with higher lipid loading. Some operators, particularly those new to the surgery, therefore find it easier to cannulate the mesenteric lymph duct when rats are pre-dosed with an oil (around 0.1 – 1 ml) such as olive or soybean oil 0.5 – 2 hr prior to commencing surgery. However, pre-dosing oil affects the lymphatic transport of lipids, lipophilic drugs and other factors in lymph such that it may not be ideal to pre-dose oil depending on the aim of the experiment.- Anesthetize the rat throughout the surgery and sample collection period.
- For example, initiate the anesthesia via subcutaneous injection of 1.5 ml/kg of Cocktail I (from step 1.2.2) into a skin fold at the back of the rats neck using a 1 ml syringe attached to a 25 G needle. Maintain the anaesthesia via intraperitoneal injection of 0.44 ml/kg of Cocktail 2 (from step 1.2.2) approximately each hour as required, using a 1 ml syringe attached to a 25 G needle.
- Prior to commencing the surgery ensure that the depth of anaesthesia is sufficient by observing respiratory rate, whisker movement, muscle tone and responses to stimuli such as pinching of foot. Administer additional anesthesia as required.
- Shave the fur from the surgical regions, which include the right side of the abdomen for lymph and duodenum cannulation, and the neck and left clavicle region for carotid artery cannulation.
- Clean the surgical regions aseptically using povidine-iodine solution or chlorhexidine solution and a 70% ethanol scrub. Repeat this 3 times for each region, finishing with a final cleansing with 70% ethanol.
- Anesthetize the rat throughout the surgery and sample collection period.
- Place the animal in dorsal recumbence on a clean sheet above a heated surgical pad (37 °C).
3. Cannulation of the Mesenteric Lymph Duct
- Place the animal with its right side facing toward the operator. Perform the surgery with or without the assistance of a surgical microscope as required.
- Open the top layer of the abdominal muscle wall with a straight 4 cm incision extending from the midline (xyphoid process) to the right flank approximately 2 cm below the ribcage (costal margin) using a sterile scalpel blade (see Figure 2B).
- Open the remaining layers of the abdominal muscle wall from 4 – 5 mm lateral to the midline to the right flank with a small pair of surgical scissors (see Figure 2B).
- Retract the small intestine under the left abdominal muscle wall and keep it in place using 2 – 3 pieces of sterile gauze saturated with normal saline.
- Bridge the rat over a 10 ml plastic syringe placed horizontally under the rat’s back at the level of right kidney to ease visualisation of the mesenteric lymph duct.
- Locate the superior mesenteric lymph duct: a vessel approximately 0.5 – 1 mm in diameter that is perpendicular to the right kidney and immediately rostral and parallel to the mesenteric artery (a pulsating dark red blood vessel; see Figure 2C).
NOTE: In non-fasted or oil pre-dosed rats the vessel is white, opaque and easier to visualise than in fasted rats in which it is quite translucent. - Inspect the area immediately caudal to the large superior mesenteric lymph duct and the mesenteric artery to determine if a second, smaller accessory lymph duct is present. If present, block the flow of lymph through the duct by severing the duct and occluding with superglue, or tying a suture around the duct, to ensure that the entire volume of lymph flowing from the intestine is collected from the superior mesenteric lymph duct.
- Pass a pair of straight forceps through the peri-renal fat bed at the lower margin of the right kidney, and through the connective tissue layers immediately below the vena cava, in a direction parallel with the superior mesenteric lymph duct.
- Using the tip of the forceps take hold of the lymph cannula and pull it through the peri-renal fat bed with one end immediately adjacent to the mesenteric lymph duct and the other exteriorised from the animal at the level of the right kidney.
- Isolate the mesenteric lymph duct from overlying layers of connective and fat tissue by blunt dissection. Take care not to pierce or damage the lymph duct.
- After isolating the lymph duct, make a small hole in the lymph duct with either micro-scissors, the sharp tip of jeweller’s forceps or a 25 G needle. Take care not to completely sever the vessel.
- Ensure that the lymph cannula is completely filled with anti-coagulant solution (using a syringe and 25 G needle) with no air gaps. Insert the lymph cannula approximately 2 – 4 mm inside the mesenteric lymph duct via the small hole with the aid of a small pair of forceps.
- Observe the lymph cannula for a couple of minutes. Observe a gradual flow of intestinal lymph from the free collecting end of the cannula if the cannulation is successful. If the cannulation is not successful, repeat steps 3.8 – 3.12.
- If the cannulation is successful, secure the cannula by placing a small drop of veterinary adhesive over the entrance hole into the lymph duct. Take care not to occlude the vessel via the application of excess veterinary adhesive.
- Whilst waiting for the veterinary adhesive to set, observe the flow of lymph for several minutes to ensure that the cannulation procedure was successful. Once certain of the success of the cannulation, remove the gauze pieces that were placed in the abdominal cavity carefully and place the small intestine in its original position.
- Place a lymph collection tube containing anti-coagulant (see step 1.6) at the end of the cannula to collect the free flowing lymph.
- Next, cannulate the duodenum for infusion of rehydration solutions and/or formulations.
4. Cannulation of the Duodenum
- Attach the duodenum cannula to a syringe (e.g., 10 ml) filled with the rehydration solution.
- Identify the duodenum as the bright pink (with more blood vessels) section of the small intestine, which upon gentle downward pulling reveals the stomach.
- Make a small puncture hole in the duodenum approximately 2 cm below the junction of the stomach and duodenum (the pylorus) using a sterile 23 G needle.
- Insert the J shaped hooked end of the duodenal cannula through the puncture hole. Secure in place with a drop of cyanoacrylate glue.
- Commence hydration of the rat by attaching the cannula to the rehydration syringe loaded in an infusion pump. According to the literature rehydration rates range from 0.5 – 3 ml/hr15,25.
- After completion of the lymph and duodenum cannulation, close the incision in the abdominal muscle wall with sutures. Then close the skin incision by applying a few drops of tissue adhesive (cyanoacrylate) along the side of the incision and pinching the skin on both sides of the incision together.
5. Cannulation of the Carotid Artery
The carotid artery cannulation may be performed before or after the cannulation of the mesenteric lymph duct. Some operators prefer to cannulate the carotid artery prior to cannulating the lymph duct so as to reduce potentially dislodging the lymph cannula when moving the animal.
- Place a mark on the carotid artery cannula 2.5 cm from the bevel tip to identify the portion of the tubing to insert into the artery. Connect the other end of the cannula (i.e., without bevel) to a 23 or 25 G needle attached to a syringe filled with anti-coagulant solution and fill the cannula with the anti-coagulant solution making sure no air gaps are present.
- To facilitate cannulation, place the anaesthetised rat in dorsal recumbency with the neck stretched and head pointing toward the operator. Note that some operators prefer to perform this technique with the rat’s tail pointing toward the operator.
- Place a 1 – 1.5 cm longitudinal incision through the skin layer above the left of the trachea in the sagittal plane.
- Dissect the subcutaneous connective tissue using blunt tip forceps to expose the underlying paired neck muscles.
- Retract the neck muscles to reveal the left carotid artery (located ~1 cm below the skin surface and pulsing). Clean the overlying connective tissue from the artery by blunt dissection.
- Isolate an ~1 cm section of the artery carefully using blunt tissue forceps, taking care not to damage the adjacent vagal nerve track. Perform this by opening and closing the blunt tip forceps vertically along either side of the artery to free it from the surrounding tissue and vagus nerve.
- Using fine tip forceps, thread two silk sutures underneath the carotid artery and place them at each end of the isolated artery section. Tie off the suture closest to the operator and furthest from the rat’s heart and clavicle (Figure 3A).
- Occlude blood flow through the artery by placing a pair of straight fine tip forceps underneath the artery (Figure 3B).
- Using the fine tip iris scissors, place a small incision on the top surface of the artery (about 1/3 of the way down from the top of the isolated area). Flush any spilled blood on the surface of the artery with heparinised saline and gently wipe using cotton tips.
- Insert the tip of the cannula into the artery with a pair of curved tip forceps. Once inserted, remove the straight fine tip forceps occluding the blood flow and advance the cannula 2.5 cm into the artery using two pairs of forceps, one to hold the cannula inside the artery to stop blood from leaking back and the other to insert the cannula.
- Use a small artery clamp to hold the cannula inside the artery and remove the straight fine tip forceps that were placed below the artery in step 5.8 to occlude the blood flow.
- Confirm the patency of the cannula by injecting anti-coagulant solution into the cannula and drawing back a small amount of blood (Figure 3C). Then flush the cannula with anticoagulant solution and seal the end using the flame of a lighter or a cannula plug.
- Tie the cannula in place with the two sutures placed under the artery in step 5.8.
- Remove the artery clamp and secure a third suture around the artery and cannula. Close the neck wound with sutures.
6. Post-surgical Period and Formulation Infusion
- Continue to maintain the rat under anaesthesia and on the heated pad.
- Rehydrate the rat via infusion of rehydration solution into the duodenum for at least 0.5 hr following completion of the cannulations. Observe the lymph flow rate decrease (~0.1 – 0.5 ml/hr) during the initial period following cannulation and soon increase to a steady baseline (0.4 – 2.5 ml/hr depending on rehydration rate).
- Following the recovery period, infuse the formulation of interest (containing lipids, drugs, etc.) into the duodenum via the cannula. Optionally, use an infusion pump to regulate the flow rate of formulation. NOTE: In the protocol described here the animals remain anaesthetised throughout the surgery and lymph collection period and are euthanised under anaesthesia such that additional analgesia is not required. If the protocol is modified and animals are enabled to regain consciousness then appropriate analgesia and post-operative care will be required.
- At the completion of formulation infusion continue to rehydrate the rat at rate of 1.5-3 ml/hr with normal saline into the duodenum to prevent dehydration.
- Continue to keep the rat on the 37˚C heated surgical pad to maintain temperature (as per step 2.3), express urine from the bladder via gentle downward push on the lower abdomen as required and reapply ophthalmic ointment every hour (as per step 2.2.3). Regular body position changes are recommended if the animal is to regain consciousness after the procedure.
7. Collection of Lymph and Blood Samples to Assess Lipid and Drug Absorption
- Collect lymph continuously into tubes containing anti-coagulant. Change tubes at the required time intervals (e.g., hourly).
- Collect blood samples at set time points.
- Occlude blood flow through the cannula by bending the cannula back approximately 2 cm from the sealed end. Unseal the cannula by cutting off the sealed end or removing the cannula plug.
- Using an empty syringe, withdraw heparinised saline from the cannula until blood fills the entire cannula.
- Using a new empty syringe, withdraw the desired volume of blood and place in a tube containing anti-coagulant.
- Using the first syringe, replace the blood taken before sampling to reduce unnecessary blood loss. Before injection, flick the syringe while holding it upright to ensure that no air bubbles enter the cannula.
- Using a syringe, replace the volume of blood removed from the rat with anti-coagulant solution. Ensure that no residual blood is visible in the cannula as any remaining may clot and block the cannula.
- Bend the cannula approximately 2 cm from the unsealed end to block blood flow and remove the syringe. Using the flame of a lighter or a cannula plug, reseal the cannula.
- At the completion of blood and lymph collection from the rat, euthanize the rat via intraperitoneal administration of >100 mg/kg sodium pentobarbitone.
- Determine lymph flow rate by measuring the mass of lymph collected during each time period.
- Measure the concentration of drug and lipid in lymph and blood using, for example, HPLC, HPLC-MS or using commercial kits, to assess lipid and drug absorption efficiency.
- Calculate mass transport of drug and lipids in lymph from the product of the measured concentrations and volume of lymph collected.
Representative Results
The results of a representative experiment to quantitate the cumulative extent and rate of lipid and drug transport via the lymphatic system following intestinal delivery using the mesenteric lymph cannulation model are shown in Figure 4 and Figure 5. In this experiment, 200 µg of the model lipophilic drug halofantrine was administered into the duodenum of rats over 2 hr in a formulation containing 40 mg oleic acid (including 2 – 5 µCi 14C-oleic acid) and 7.1 mg 2-monoolein dispersed in 5.6 ml of 5 mM sodium taurocholate in phosphate buffered saline pH 6.9. Following formulation administration the rats were rehydrated at a rate of 2.8 ml/hr with normal saline infused into the duodenum. Lymph was collected continuously into tubes changed each hour for 10 hr. The formulation was prepared, and the experiment performed, according to a protocol described previously for a formulation containing all the same components except it did not include 2-monoolein43.
As shown in Figure 4, in these experimental conditions the mean lymph flow rate for successfully cannulated rats was between 0.4 – 1.3 ml/hr. In some rats the lymph flow rate was slightly lower in the first 1 – 3 hr following cannulation and then increased. This is commonly seen following lymph cannulation. Also shown in Figure 4 is the lymph flow rate for an unsuccessful experiment. In this case the flow rate remained substantially lower than that seen in the successful experiments throughout the collection period. Similarly, the cumulative lymphatic transport of triglyceride and halofantrine was substantially lower in the unsuccessful experiment (Figure 5A and B). The mean cumulative halofantrine transport in the successful group was 15.1% of the dose, the cumulative triglyceride transport was 61 mg over 10 hr and the proportion of the administered radiolabelled exogenous lipid dose transported in lymph was 54% (Figure 5C). These values are not significantly different to that seen previously for a similar formulation that contained the same components except for the absence of 2-monoolein43 (Table 1). This suggests that formulations containing fatty acids in the absence of a source of monoglyceride can support similar lipid and drug transport in lymph to those which do contain monoglyceride. This result is described further in the discussion section.
Figure 5D shows representative data for the rate of halofantrine and triglyceride transport in lymph over time following administration. The transport rate of both triglyceride and drug into lymph peaks a couple of hours following lipid and drug administration and then returns to baseline levels. As is typical in intestinal lymphatic lipid and drug transport experiments, the rate of drug transport in lymph during each time period mirrors that seen for triglyceride transport as drug is transported into lymph in association with the triglyceride-rich lipoproteins.

Figure 1. Diagrammatic representation of the shape of the beveled tip of a carotid artery or lymph cannula, and the J-shaped beveled tip of a duodenal cannula.
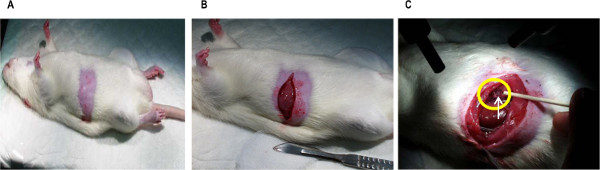
Figure 2. Photographs of (A) the abdomen shaved in preparation to cannulate the lymph duct, (B) the abdominal muscle wall opened with a straight 4 cm incision extending from the midline to the right flank to access the lymph duct, and (C) the superior mesenteric lymph duct (in yellow circle) perpendicular to the right kidney. Please click here to view a larger version of this figure.
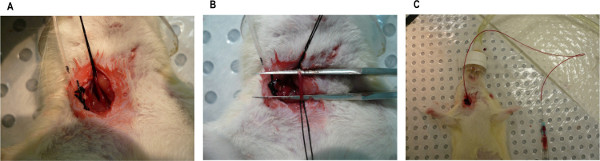
Figure 3. Photographs of (A) the carotid artery isolated with two silk sutures with the one closest to the operator tied off, (B) the carotid artery isolated and blood flow occluded with a pair of straight fine tip forceps placed underneath the artery, and (C) a carotid artery with cannula in place and the patency being checked by drawing back a small amount of blood. Please click here to view a larger version of this figure.
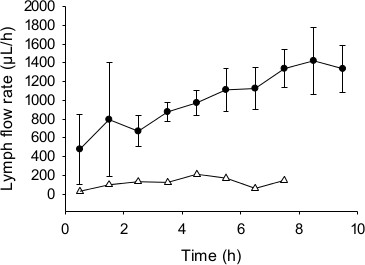
Figure 4. Representative data for mesenteric lymph flow rate (µl/hr) over time. Rats were administered a formulation containing 200 µg halofantrine, 40 mg oleic acid (with 2 µCi 14C-oleic acid) and 7.1 mg 2-monoolein in 5.6 ml of 5 mM sodium taurocholate in phosphate buffered saline (pH 6.9) from 0 – 2 hr. Data shown are the mean ± SEM for n = 3 successful experiments (closed circles, ●) and n = 1 unsuccessful outcome (open triangles, ∆).
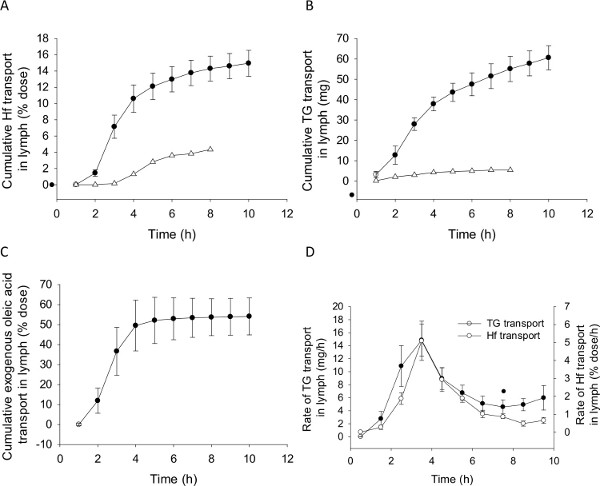
Figure 5. Representative data for lipid and drug transport into mesenteric lymph. Cumulative transport of (A) the model drug halofantrine (Hf, % dose), (B) triglyceride (TG, mg) and (C) exogenous fatty acid (% dose), and the rate of transport of (D) TG (mg/hr) and Hf (% dose/hr) into mesenteric lymph over time following administration of a formulation containing 200 µg halofantrine, 40 mg oleic acid (with 2 µCi 14C-oleic acid) and 7.1 mg 2-monoolein in 5.6 ml of 5 mM sodium taurocholate in phosphate buffered saline (pH 6.9) from 0 – 2 hr. Data shown are the mean ± SEM for n = 4 successful experiments (closed circles, ●) and n = 1 unsuccessful outcome (open triangles, ∆). Exogenous fatty acid transport was not measured in the unsuccessful experiment but is typically low in failed experiments. Data for the unsuccessful experiment is omitted from Panel D for clarity. Please click here to view a larger version of this figure.
| Without monoolein | With 2-monolein | |||
| Mean | SEM | Mean | SEM | |
| Halofantrine (% dose) | 15.1 | 0.8 | 15 | 1.6 |
| Triglyceride (mg) | 67 | 4 | 61 | 6 |
| Total fatty acid (µmol) | 261 | 16 | 248 | 23 |
| Exogenous oleic acid (µmol) | 82 | 5 | 65 | 6 |
| Endogenous fatty acid (µmol) | 180 | 17 | 183 | 28 |
Table 1. Comparison of cumulative lymphatic transport data over 10 hr following administration of 200 µg halofantrine to mesenteric lymph duct cannulated rats in formulations containing 40 mg oleic acid (containing 1 µCi 14C-oleic acid) emulsified in 5 mM sodium taurocholate in phosphate buffered saline (pH 6.9) with and without 7.1 mg 2-monoolein. Data represent mean ± SEM for n = 4 rats. No statistically significant differences are seen between the 2 groups.
aIn the group dosed with 2-monoolein this is not an accurate measure of endogenous fatty acid transport as the oleic acid attached to the 2-monoolein backbone is not accounted for in the measured exogenous oleic acid transport into lymph.
bThe data for halofantrine and total fatty acid transport in the group dosed without 2-monoolein was previously published in Figure 5 of reference 43 and is reproduced here in table format.
Discussion
The rat mesenteric lymph cannulation model enables direct quantification of the concentration and rate of transport of various cells and molecules (such as lipids and drugs) from the intestine into the lymph and the changes to these that occur in response to challenge of various substances (diet, antigen, drugs, formulations, etc.)10,27 and disease (cancer, viruses, colitis, insulin resistance, etc.)5-7. The components collected in lymph may also be further used in additional experiments. For example, cells can be cultured44, lipoproteins fractionated and lymph or its components such as lipoproteins or cells, loaded with markers including drugs, radiolabels and fluorescent probes. These can then be re-infused into recipient animals to more directly evaluate their function, metabolism, clearance and/or tissue disposition patterns45-47. The rat lymph cannulation model has several advantages over the other in vitro, in situ, in silico, and in vivo models that were described in the introduction. Most importantly, cannulation is the only method which enables direct access to the entire volume of components in lymph fluid. When performed in the hands of an experienced operator the model is robust, reproducible and experimental success rates of >80% can be achieved. However, the surgical technique can be difficult to initially master, particularly in a laboratory that is not familiar with the technique.
Several steps are critical to success of the mesenteric lymph cannulation model. The first is the correct choice of surgical instruments and cannula type and preparation. Our lab uses PE cannulas with dimensions OD 0.8 x ID 0.5 mm. However, other labs have experienced success with PVC cannulas of the same dimension15. Bevelling the tip of the cannula and pre-soaking it in an anti-coagulant also helps to prevent occlusion of, and the formation of clots within, the cannula post-insertion (step 3.13). The first step of the surgical procedure that some operators find particularly critical is taking care to clean the layers of connective and fat tissue above the lymph duct (step 3.10). This helps to avoid the insertion of the cannula between the tissue and lymph duct rather than into the lymph duct. However, this cleaning step is difficult as the lymph duct is fragile and easy to damage. For some, this step and the insertion of the cannula are most successfully completed with the aid of a surgical microscope although others find a microscope is not necessary. When inserting the cannula into the duct the direction and depth of insertion relative to the bevelled tip requires some consideration in order to prevent the cannula being occluded by the vessel wall (step 3.12). Each operator tends to find their own individual preference. Also important is the avoidance of air bubble formation within the cannula during insertion as air gaps apply back pressure to the free flow of lymph (step 3.12). Finally, the application of veterinary adhesive to secure the cannula in place should be below the point of insertion into the duct as adhesive placed directly on top of the duct can cause the vessel to collapse and occlude the cannula tip (step 3.14).
The easiest initial guide as to whether a surgery is successful is the flow rate of lymph. Once the cannula is in place lymph generally begins to flow slowly in the first 30 min and then increases. Typically, flow rates for successful cannulations in rats >250 g are >0.1 ml/hr in the first hour and then increase to >0.4 ml/hr at steady state as is shown in Figure 4. Although of course this may vary depending on the experimental condition. In terms of troubleshooting, if no lymph is flowing through the cannula or the flow rate is low this may be because:
- the cannula was not inserted correctly into the duct
- the cannula is in the duct but occluded by the side of the vessel wall
- the cannula is in the duct but occluded by adhesive applied in the incorrect position
- the cannula is in the duct and an air bubble in the cannula is slowing the flow
- a clot has formed within the cannula
Careful inspection usually reveals the underlying factor. If the operator believes the cannula was not placed correctly in the first place (i.e., lymph was never flowing) then re-insertion is necessary. If the cannula appears to be in the correct place then the first factor to check is whether an air bubble or clot is present. Air bubbles will generally pass through the cannula as lymph flows and temporarily slow flow. It is sometimes possible to remove air bubbles or clots by drawing back on the cannula using a 25 G needle attached to, for example, a 1 ml syringe. If there is no air bubble or clot it can be useful to try re-positioning the rat and/or turning or pulling the cannula slightly. This can sometimes realign the cannula such that it is not blocked against the vessel wall. Occasionally removing the adhesive with or without small movement of the cannula enables the lymph to flow again also. However, if all else fails the cannula needs to be reinserted. In our experience experiments are less successful when the cannula was not inserted correctly on the first attempt. However, surgical rescue is possible. Another complication that can arise is difficulty in cannulation due to anatomical variance between rats. For example, the mesenteric lymph duct occasionally passes in an awkward direction or there is an accessory lymph duct on the opposite side of the mesenteric artery to the larger superior mesenteric lymph duct. In experiments which require collection of the entire volume of lymph flowing from the small intestine (as is the case when assessing total lipid and drug transport from the intestine via the lymphatics), the accessory lymph duct needs to be occluded such that all lymph is directed to the superior lymph duct. This is difficult to achieve but can be attempted by tying a suture around the accessory duct or severing the accessory duct and occluding it with veterinary adhesive (step 3.6). The presence of an accessory lymph duct is one of the main factors that results in experimental failure in lymphatic drug transport experiments. If the accessory vessel is not completely occluded, lymph flow and lipid and drug transport are significantly lower than expected.
In the protocol presented here the animal remains anaesthetised throughout the lymph collection period. The anaesthetised rat model (rather than conscious models described below) increases experimental throughput as the surgery and experiment can be conducted over one rather than several days and the surgical success rate is greater as it is easier to maintain cannula patency in immobilized animals. Anaesthesia can theoretically reduce gastric emptying, intestinal lipid processing and lymph flow and transport. In our experience, however, lymphatic lipid and drug transport are similar in anaesthetised rats administered the drug directly into the duodenum (to bypass stomach emptying) within pre-digested and pre-dispersed vehicles (e.g., fatty acid and monoglyceride micellar systems dispersed with surfactants) when compared to conscious rats administered the drug via oral gavage into the stomach in an equivalent triglyceride21,23,27. Indeed, the majority of intestinal lymphatic drug transport experiments in our lab in the last 10 years have been conducted in the anaesthetised model due to the higher throughput that can be achieved. In these experiments we have most often administered drugs in formulations containing fatty acid (e.g., oleic acid) and surfactant43. Fatty acids are synthesised into triglycerides prior to incorporation into intestinal lymph lipoproteins and this requires the source of components to form the glycerol backbone of triglyceride (ie 2-monoglyceride or glycerol-3-phosphate). This suggests that administration of fatty acids in the absence of a glycerol source may lead to reduced lymphatic transport. Administration of fatty acid, however, enables the direct calculation of the contribution of the exogenous administered fatty acid and endogenous lipids to lymphatic lipid and drug transport43. Additionally, 2-monoglyceride is expensive and generally unstable as it readily isomerises to 1-monoglyceride which is digested to glycerol in the intestinal lumen. In the studies reported here we further demonstrate that lymphatic lipid and drug transport is equivalent following administration of the model drug halofantrine with a fatty acid (e.g., oleic acid) and surfactant (bile salt) formulation in either the presence or absence of the glycerol source 2-monoolein (Table 1). Endogenous sources of glycerol thus appear sufficient to support equivalent lymph flow and lipid and drug transport following administration of this model drug with fatty acids (e.g., oleic acid) in the absence of a glycerol source. This provides confidence that representative lymphatic transport data can be obtained with simple fatty acid formulations.
The anaesthetised model is readily extended to a conscious model when required. In the conscious models formulations can be gavaged directly into the stomach or infused into the duodenum or intravenously, direct comparisons can be made to pharmacokinetic studies conducted in non-lymph cannulated conscious animals and results may be considered more physiologically relevant. The longer time period allowed in conscious animals also has the advantage of allowing the collection of more complete pharmacokinetic profiles for drug concentrations in blood, whereas in anaesthetised experiments, blood profiles are often incomplete, especially for long half life drugs. As described above, however, the success rate for conscious lymph cannulated studies is lower and experiments take longer to complete. There are two types of conscious lymph cannulation models described in the literature. In conscious restrained models15, following the lymph cannulation surgery, the animal is simply turned onto its front and placed in an appropriate restraint for the remainder of the experiment with the lymph cannula externalised and inserted into a collection tube. The animal is then allowed to regain consciousness and recover from the surgical procedure O/N. The success rate with this model is very good in the hands of experienced operators but it can be difficult to obtain ethics clearance to restrain animals for the prolonged period of time required for lymph collection. An alternate model is where lymph is collected from conscious and freely moving rats. This model has been detailed previously25. In this model long cannulas are inserted into the mesenteric lymph duct, duodenum and carotid artery. The cannulas are then tunnelled below the skin, exteriorized at the back of the neck and placed through a swivel system. The rat is placed in a harness attached to the swivel and allowed to regain consciousness and to recover from the surgical procedure O/N. The animal has free movement within a metabolic cage and lymph and blood samples can be collected from the cannulas externalised outside the cage.
Mesenteric lymph cannulation remains the only tool which enables the direct evaluation of lymph components in their nascent state. Lymph cannulation is most often described in rats as the surgery is less complex than smaller and large animals, the model less expensive than large animals and results appear reasonably comparable across species27. The rat model can be modified according to experimental need and the success rate is high in the hands of experienced operators. The model can also be coupled with other in vitro or in vivo studies to examine, in detail, the metabolism or function of intestinal lymph components. Once established the mesenteric lymph cannulated rat model is thus a powerful tool to evaluate the concentration, transport, function and metabolism of various parameters that pass directly from the intestine to the lymphatic system (and in many cases flow eventually to the systemic circulation).
Disclosures
The authors have nothing to disclose.
Acknowledgements
Funding from the Australian Research Council (ARC) and National Health and Medical Research Council (NHMRC) is gratefully acknowledged.
Materials
| Sterile saline | Baxter healthcare | AHB 1307 | Any brand can be used. Example here is Baxter 100 ml saline bags, box of 50 |
| 70 % ethanol in water | Any | Any brand can be used | |
| Chlorhexidine gluconate solution (Microshield 4) | Livingstone International | JJ60243L | Any brand can be used. http://www.livingstone.com.au/?PG=search_result&CAT=6&search =JJ60243L |
| Betadine solution | Livingstone International | BU0510 | Any brand can be used. http://www.livingstone.com.au/?PG=search_result&CAT=6&search =BU0510 |
| Ilium Ketamil (Ketamine 100 mg/ml) | PROVET VICTORIA | KETA I 1 | http://www.provet.com.au/ |
| Ilium Xylazil (Xylazine 100 mg/ml) | PROVET VICTORIA | TRO-3828 | http://www.provet.com.au/ |
| ACP 10 Injection (Acepromazine 10 mg/ml) | PROVET VICTORIA | VTG-DACP010020 | http://www.provet.com.au/ |
| Sodium pentobarbitone | PROVET VICTORIA | 24529 | Any brand can be used. Example here is Lethabarb® 325 mg/ml sodium pentobarbitone, Virbac Animal Health. http://www.provet.com.au |
| Heparin (35000I.U. in 35 mL) | Sigma Pharmaceuticals | 337220 | http://sigmaco.com.au/ |
| Ethylenediaminetetraacetic acid (EDTA) disodium salt dihydrate | Sigma-Aldrich | E1644 | Any brand can be used. Example here is disodium salt of EDTA from Sigma. |
| Polyethylene (PE) cannula o.d. 0.96 mm x i.d. 0.58 mm | Microtube extensions | PE8050 | Any brand can be used. Example here is PE tubing 0.8×0.5 mm, 30 m |
| Polyethylene (PE) cannula o.d. 0.8 mm x i.d. 0.5 mm | Microtube extensions | PE9658 | Any brand can be used. Example here is PE tubing 0.96×0.58 mm, 30 m |
| Ruler | Any | Any brand can be used | |
| Markers | Any | Any brand can be used | |
| Cigarette lighter | Any | Any brand can be used | |
| Cyanoacrylate glue | Any | Any brand can be used | |
| 23 gauge needles | Livingstone International | DN23GX0.75LV | Any brand can be used. Example here is Livingstone Disposable Needle, Sterile, 23GX0.75inch, 100/BOX. http://www.livingstone.com.au/?PG=search_result&CAT= 6&search=DN23GX0.75LV |
| 25 gauge needles | Livingstone International | DN25GX1.0LV | Any brand can be used. Example here is Livingstone Disposable Needle, Sterile, 25GX1.0inch, 100/BOX. http://www.livingstone.com.au/?PG=search_result&CAT=6&search= DN25GX1.0LV |
| 1 ml syringe | Livingstone International | T3SS01TA | Any brand can be used. Example here is Terumo syringe 1 ml Slip Tuberculin 100/Box. http://www.livingstone.com.au/?PG=search_result&CAT=6&search =T3SS01TA |
| 10 ml syringe | Livingstone International | T3SS10SA | Any brand can be used. Example here is Terumo syringe 10 ml Slip 100/Box. http://www.livingstone.com.au/?PG=search_result&CAT=6&search =T3SS10SA |
| Gauze swabs | Livingstone International | GSC075 | Any brand can be used and cut to required size. Example here is gauze swabs cotton filled 7.5×7.5 cm, 8 ply. http://www.livingstone.com.au/?PG=search_result&CAT=6&search =GSC075 |
| Cotton buds | Livingstone International | CTAST075DP | Any brand can be used. Example here is Livingstone cotton applicator plastic double tipped. 75MM. 100/PK. http://www.livingstone.com.au/?PG=search_result&CAT=6&search =CTAST075DP |
| Heating pad | Ratek | WT1 | Any brand that keeps temperature at 37C can be used. Example here is Ratek warming tray. |
| Surgical light | Harvard Apparatus | 72-0215 with 72-0267 | Any brand can be used. Example here is Harvard apparatus V-Lux 1000 Cold Light Source with Bifurcated Gooseneck Light Guide, Black, 4.7 mm fiber diameter (each arm). http://www.harvardapparatus.com/webapp/wcs/stores/servlet/product_11051_10001_50601_ -1_HAI_ProductDetail and http://www.harvardapparatus.com/webapp/wcs/stores/servlet/product_11051_10001_35487_ -1_HAI_ProductDetail___ |
| Surgical microscope | Zeiss | 495005-0014-000 | Any brand can be used. Example here is Zeiss Stereomicroscope Stemi 2000-C with Stand S Double Spot and KL 300 LED. https://www.micro-shop.zeiss.com/?l=en&p=us&f=e&i=10143 |
| Silk suture | Livingstone International | DTSK163019F4 | Any brand can be used. Example here is * Email this item to my friend 3/8 Circle Reverse Cut Silk Suture 3/0 Thread 19mm. http://www.livingstone.com.au/?PG=search_result&CAT=6&search =DTSK163019F4 |
| Scalpel blades | Fine Science Tools (FST) | 10020-00 | Any brand can be used. Example here is FST Scalpel Blade #20. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=191 |
| Scalpel handle | Fine Science Tools (FST) | 10004-13 | Any brand can be used. Example here is FST Scalpel Handle #4. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=298&CategoryId=51 |
| 1 x Small surgical scissors | Fine Science Tools (FST) | 14060-09 | Any brand can be used. Example here is FST Fine Scissors, 9 cm with 21 mm cutting edge, sharp, straight. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=40&CategoryId=17 |
| 2 x Forceps with serrated curved tip | Fine Science Tools (FST) | 11001-13 | Any brand can be used. Example here is FST 13 cm standard pattern forceps with curved 2.8×1.4 mm tip. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=405&CategoryId=32 |
| 1 x Iridectomy scissors | Fine Science Tools (FST) | 15000-08 | Any brand can be used. Example here is FST Vannas Spring Scissors – 2.5mm Cutting Edge, Straight. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=17&CategoryId=16 |
| 1 x Forceps with straight serated tip | Fine Science Tools (FST) | 11650-10 | Any brand can be used. Example here is FST Graefe 10 cm straight with serrated 1 x 0.99 mm tip. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=390&CategoryId=32 |
| 1 x Forceps with smooth sharp straight fine tip | Fine Science Tools (FST) | 11251-10 | Any brand can be used. Example here is FST Dumont #5 forceps straight 11cm with 0.08 x 0.04mm tip. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=335&CategoryId=29 |
| 1 x Forceps with smooth fine curved forceps | Fine Science Tools (FST) | 11063-07 | Any brand can be used. Example here is FST Delicate Forceps 9 cm with smooth 0.4 x 0.3mm tip. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=360 |
| 2 x Hemostats | Fine Science Tools (FST) | 13010-12 | Any brand can be used. Not all operators use the hemostats. Example here is FST 12 cm Micro-Mosquito Hemostats with 20 mm length x 1.3 mm width serrated, straight tip. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=377&CategoryId=33 |
| 1 x Suture needle holder | Fine Science Tools (FST) | 12001-13 | Any brand can be used. Example here is FST 13cm Hasley Needle Holder with 16 mm length x 1.9 mm width tip. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=254&CategoryId=70 |
| 1 x Artery clamp | Fine Science Tools (FST) | 18050-28 | Any brand can be used. Example here is FST Bulldog Serrefines straight, 28 mm long, 9×1.6 mm jaw dimension with medium clamp press. http://www.finescience.ca/Special-Pages/Products.aspx?ProductId=270&CategoryId=82 |
| Oleic acid | Sigma Aldrich | O1008 | When required, any brand can be used. Example here is 99% pure oleic acid. http://www.sigmaaldrich.com/catalog/product/sial/o1008?lang=en®ion=AU |
| 14C-oleic acid | Perkin | NEC317050UC | Any brand can be used. Example here is Oleic Acid, [1-14C]-, 50µCi (1.85MBq). http://www.perkinelmer.com/Catalog/Product/ID/NEC317050UC |
| Sodium taurocholate | Sigma Aldrich | T4009 | Any brand can be used. Example here is taurocholic acid sodium salt hydrate ≥95% (TLC) . http://www.sigmaaldrich.com/catalog/product/sigma/t4009?lang=en®ion=AU |
| Halofantrine | Glaxo Smith Kline | Halofantrine was kindly provided as a gift from Glaxo Smith Kline | |
| Sodium phosphate monobasic | Sigma Aldrich | 71507 | Any brand can be used. Example here is sodium phosphate monobasic monohydrate, BioXtra, for molecular biology, >99.5%. http://www.sigmaaldrich.com/catalog/product/sigma/71643?lang=en®ion=AU |
| Sodium phosphate dibasic | Sigma Aldrich | 71643 | Any brand can be used. Example here is sodium phosphate dibasic dihydrate, BioUltra, for molecular biology, >99%. http://www.sigmaaldrich.com/catalog/product/sigma/71507?lang=en®ion=AU |
References
- Barrowman, J. A., Tso, P. Gastrointestinal lymphatics. Comprehensive Physiology. , 1733-1777 (2010).
- Karaman, S., Detmar, M. Mechanisms of lymphatic metastasis. J Clin Invest. 124 (3), 922-928 (2014).
- Mossel, E. C., Ramig, R. F. A lymphatic mechanism of rotavirus extraintestinal spread in the neonatal mouse. J Virol. 77 (22), 12352-12356 (2003).
- Pantaleo, G., et al. Hiv-Infection Is Active and Progressive in Lymphoid-Tissue during the Clinically Latent Stage of Disease. Nature. 362 (6418), 355-358 (1993).
- Chakraborty, S., Zawieja, S., Wang, W., Zawieja, D. C., Muthuchamy, M. Lymphatic system: a vital link between metabolic syndrome and inflammation. Annals of the New York Academy of Sciences. 1207, R94-R102 (2010).
- Dixon, J. B. Lymphatic lipid transport: sewer or subway. Trends Endocrinol Metab. 21 (8), 480-487 (2010).
- Weid, P. -. Y., Rehal, S., Ferraz, J. G. Role of the lymphatic system in the pathogenesis of Crohn’s disease. Current Opinion in Gastroenterology. 27 (4), 335-341 (2011).
- Wang, Y., et al. Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice. PloS one. 4 (12), e8442 (2009).
- Ji, Y., et al. Activation of rat intestinal mucosal mast cells by fat absorption. Am J Physiol Gastrointest Liver Physiol. 302 (11), G1292-G1300 (2012).
- Kohan, A., Yoder, S., Tso, P. Lymphatics in intestinal transport of nutrients and gastrointestinal hormones. Ann N Y Acad Sci. 1207, E44-E51 (2010).
- Trevaskis, N. L., Charman, W. N., Porter, C. J. Targeted drug delivery to lymphocytes: a route to site-specific immunomodulation. Mol Pharm. 7 (6), 2297-2309 (2010).
- Rothkotter, H. J., Huber, T., Barman, N. N., Pabst, R. Lymphoid cells in afferent and efferent intestinal lymph: lymphocyte subpopulations and cell migration. Clin Exp Immunol. 92 (2), 317-322 (1993).
- Trevaskis, N. L., Charman, W. N., Porter, C. J. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 60 (6), 702-716 (2008).
- Mansbach, C. M., Dowell, R. F., Pritchett, D. Portal transport of absorbed lipids in rats. Am J Physiol. 261 (3 Pt 1), G530-G538 (1991).
- Kohan, A. B., Howles, P. N., Tso, P. Methods for studying rodent intestinal lipoprotein production and metabolism. Curr Protoc Mouse Biol. 2, 219-230 (2012).
- Porter, C. J., Trevaskis, N. L., Charman, W. N. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 6 (3), 231-248 (2007).
- Trevaskis, N. L., et al. The role of the intestinal lymphatics in the absorption of two highly lipophilic cholesterol ester transfer protein inhibitors (CP524,515 and CP532,623). Pharm Res. 27 (5), 878-893 (2010).
- Choo, E. F., et al. The Role of Lymphatic Transport on the. Systemic Bioavailability of the Bcl-2 Protein Family Inhibitors Navitoclax (ABT-263) and ABT-199. Drug Metabolism and Disposition. 42 (2), 207-212 (2014).
- Han, S., et al. Targeted delivery of a model immunomodulator to the lymphatic system: comparison of alkyl ester versus triglyceride mimetic lipid prodrug strategies. J Control Release. 177, 1-10 (2014).
- Bollman, J. L., Cain, J. C., Grindlay, J. H. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med. 33 (10), 1349-1352 (1948).
- Porter, C. J., Charman, S. A., Charman, W. N. Lymphatic transport of halofantrine in the triple-cannulated anesthetized rat model: effect of lipid vehicle dispersion. J Pharm Sci. 85 (4), 351-356 (1996).
- Boyd, M., Risovic, V., Jull, P., Choo, E., Wasan, K. M. A stepwise surgical procedure to investigate the lymphatic transport of lipid-based oral drug formulations: Cannulation of the mesenteric and thoracic lymph ducts within the rat. Journal of Pharmacological and Toxicological Methods. 49 (2), 115-120 (2004).
- Porter, C. J., Charman, S. A., Humberstone, A. J., Charman, W. N. Lymphatic transport of halofantrine in the conscious rat when administered as either the free base or the hydrochloride salt: effect of lipid class and lipid vehicle dispersion. J Pharm Sci. 85 (4), 357-361 (1996).
- Caliph, S. M., Charman, W. N., Porter, C. J. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J Pharm Sci. 89 (8), 1073-1084 (2000).
- Edwards, G. A., Porter, C. J., Caliph, S. M., Khoo, S. M., Charman, W. N. Animal models for the study of intestinal lymphatic drug transport. Adv Drug Deliv Rev. 50 (1-2), 45-60 (2001).
- Noguchi, T., Charman, W. N. A., Stella, V. J. Lymphatic Appearance of Ddt in Thoracic or Mesenteric Lymph Duct Cannulated Rats. International Journal of Pharmaceutics. 24 (2-3), 185-192 (1985).
- Trevaskis, N. L., et al. A mouse model to evaluate the impact of species, sex, and lipid load on lymphatic drug transport. Pharm Res. 30 (12), 3254-3270 (2013).
- Kota, J., et al. Lymphatic absorption of subcutaneously administered proteins: influence of different injection sites on the absorption of darbepoetin alfa using a sheep model. Drug Metab Dispos. 35 (12), 2211-2217 (2007).
- McHale, N. G., Adair, T. H. Reflex modulation of lymphatic pumping in sheep. Circ Res. 64 (6), 1165-1171 (1989).
- White, D. G., Story, M. J., Barnwell, S. G. An Experimental Animal-Model for Studying the Effects of a Novel Lymphatic Drug Delivery System for Propranolol. International Journal of Pharmaceutics. 69 (2), 169-174 (1991).
- Khoo, S. M., Edwards, G. A., Porter, C. J., Charman, W. N. A conscious dog model for assessing the absorption, enterocyte-based metabolism, and intestinal lymphatic transport of halofantrine. J Pharm Sci. 90 (10), 1599-1607 (2001).
- Kararli, T. T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 16 (5), 351-380 (1995).
- Kassis, T., et al. Dual-channel in-situ optical imaging system for quantifying lipid uptake and lymphatic pump function. J Biomed Opt. 17 (8), 086005 (2012).
- Dahan, A., Hoffman, A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur J Pharm Sci. 24 (4), 381-388 (2005).
- Xiao, C., Lewis, G. F. Regulation of chylomicron production in humans. Biochim Biophys Acta. 1821 (5), 736-746 (2012).
- Seeballuck, F., Ashford, M., O’Driscoll, C. The Effects of Pluronic® Block Copolymers and Cremophor EL on Intestinal Lipoprotein Processing and the Potential Link with P-Glycoprotein in Caco-2 Cells. Pharmaceutical Research. 20 (7), 1085-1092 (2003).
- Levy, E., Mehran, M., Seidman, E. Caco-2 cells as a model for intestinal lipoprotein synthesis and secretion. The FASEB Journal. 9 (8), 626-635 (1995).
- Cartwright, I. J., Higgins, J. A. Isolated rabbit enterocytes as a model cell system for investigations of chylomicron assembly and secretion. Journal of Lipid Research. 40 (7), 1357-1365 (1999).
- Dixon, J. B., Raghunathan, S., Swartz, M. A. A Tissue-Engineered Model of the Intestinal Lacteal for Evaluating Lipid Transport by Lymphatics. Biotechnology and Bioengineering. 103 (6), 1224-1235 (2009).
- Gershkovich, P., et al. The role of molecular physicochemical properties and apolipoproteins in association of drugs with triglyceride-rich lipoproteins: in-silico prediction of uptake by chylomicrons. Journal of Pharmacy and Pharmacology. 61 (1), 31-39 (2009).
- Gershkovich, P., Hoffman, A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: Linear correlation with intestinal lymphatic bioavailability. European Journal of Pharmaceutical Sciences. 26 (5), 394-404 (2005).
- Holm, R., Hoest, J. Successful in silico predicting of intestinal lymphatic transfer. International Journal of Pharmaceutics. 272 (1-2), 189-193 (2004).
- Trevaskis, N. L., Porter, C. J., Charman, W. N. Bile increases intestinal lymphatic drug transport in the fasted rat. Pharm Res. 22 (11), 1863-1870 (2005).
- Miura, S., et al. Increased proliferative response of lymphocytes from intestinal lymph during long chain fatty acid absorption. Immunology. 78 (1), 142-146 (1993).
- Caliph, S. M., et al. The impact of lymphatic transport on the systemic disposition of lipophilic drugs. J Pharm Sci. 102 (7), 2395-2408 (2013).
- Caliph, S. M., Trevaskis, N. L., Charman, W. N., Porter, C. J. Intravenous dosing conditions may affect systemic clearance for highly lipophilic drugs: implications for lymphatic transport and absolute bioavailability studies. J Pharm Sci. 101 (9), 3540-3546 (2012).
- Trevaskis, N. L., et al. Tissue uptake of DDT is independent of chylomicron metabolism. Arch Toxicol. 80 (4), 196-200 (2006).

