Quantitation of Endothelial Cell Adhesiveness In Vitro
Summary
We report an in vitro method that allows the quantitation of the actual number of adhesive cells within an endothelial cell monolayer.
Abstract
One of the cardinal processes of inflammation is the infiltration of immune cells from the lumen of the blood vessel to the surrounding tissue. This occurs when endothelial cells, which line blood vessels, become adhesive to circulating immune cells such as monocytes. In vitro measurement of this adhesiveness has until now been done by quantifying the total number of monocytes that adhere to an endothelial layer either as a direct count or by indirect measurement of the fluorescence of adherent monocytes. While such measurements do indicate the average adhesiveness of the endothelial cell population, they are confounded by a number of factors, such as cell number, and do not reveal the proportion of endothelial cells that are actually adhesive. Here we describe and demonstrate a method which allows the enumeration of adhesive cells within a tested population of endothelial monolayer. Endothelial cells are grown on glass coverslips and following desired treatment are challenged with monocytes (that may be fluorescently labeled). After incubation, a rinsing procedure, involving multiple rounds of immersion and draining, the cells are fixed. Adhesive endothelial cells, which are surrounded by monocytes are readily identified and enumerated, giving an adhesion index that reveals the actual proportion of endothelial cells within the population that are adhesive.
Introduction
Infiltration of immune cells such as monocytes across the endothelial cell layer that line blood vessels is an important step in the process of inflammation1. This allows the homing of immune cells (immunocytes) to a site of injury. In other instances and locations such as the coronary artery and carotid artery, infiltration of monocytes through the endothelial layer can lead to the undesirable long term residence of these cells in the wall of the artery, potentially leading to the formation of plaques2. In all cases of immunocyte infiltration, the first step involves the activation of the endothelial cells in a localized region of the blood vessel. Endothelial cells are activated by pro-inflammatory cytokines such as TNF-α and IL-6 to increase expression of cell surface proteins such as VCAM, ICAM and E-selectin which facilitate the recruitment and attachment of the immunocytes onto the endothelial cell surface3-6.
Initially, measurement of endothelial cell adhesion was carried out by counting monocytes that adhered to endothelial monolayer 7. The limitations of this method in terms of precision led to the use of radio-labeled lymphocyte or monocyte, followed by quantification of radioactive material which corresponds to lymphocytes or monocytes adhesion 4. This method was eventually superseded by a fluorescence-based method, whereby immunocytes of interest were labeled with fluorescence dye and subjected to the same procedure as above with the difference being that fluorescence instead of radioactivity is measured 8. Presently this method has emerged as the most convenient and is sold in kit form by several commercial suppliers. While this assay can measure relative levels of adhesiveness between controls and experimental samples, it does not reveal whether a change in fluorescence is due to a uniform change in adhesiveness across the entire endothelial cell population or if the change is due to a difference of adhesiveness within a sub-population of cells. It is also evident that the stringency of washing exerts a profound effect on the result, and more importantly, the uniformity of rinsing off unbound monocytes between different endothelial cell monolayers will impact greatly on the closeness of results from replicates and reproducibility of the results between samples. More recently, several systems that pump monocytes in media across an endothelial cell monolayer were used to address this problem 9. In addition, these flow systems also recapitulate the effect of shear force on the endothelial cells. While the advantages of such systems are clear and very attractive, it is also important to realize that while endothelial cell adhesiveness can be greatly augmented, as is the case in acute inflammation, by factors such as TNFα, some other activators, such as ionizing radiation10 elicit changes that are not readily detected within in vitro experimental time frames by these very stringent systems. While such minute changes of endothelial cell adhesiveness are easily missed or dismissed in vitro, it is not necessarily benign in vivo, where such small changes within a life time, which is characteristic of chronic inflammation, can exert very significant outcomes. Hence a robust, yet sensitive, and specific method to detect and measure endothelial cell adhesiveness is needed.
Here, we report a method to measure adhesiveness of endothelial cells directly. This method does not rely on fluorescence measurement as an indirect surrogate indicator of endothelial cell adhesiveness. It reveals whether changes in adhesiveness are due to uniform change across all cells or confined to a sub-population. Furthermore, it allows co-staining of endothelial cells with markers such as senescence-associated beta-galactosidase, cell viability marker, Calcien AM and antibodies against cell surface and intracellular proteins, allowing the association of individual adhesive endothelial cells to certain cell states or expression of specific proteins.
Protocol
1. Preparation of Glass Coverslips
- Sterilize 12 mm diameter round glass coverslips by soaking them in 70% ethanol for at least 10 min with occasional agitation to ensure total exposure of all coverslips to the ethanol. Pour glass coverslips and ethanol into a sterile cell culture dish (either 6 cm or 10 cm diameter).
- With a pair of sterile fine 5B forceps, pick up and place each individual glass coverslip into a well of a 24 well cluster plate. Take care to ensure that the coverslips are not touching the sides of the well and are approximately in the middle of the well.
- Leave the un-covered 24 well cluster plate with glass coverslips to dry in the flow cabinet. This takes about 10 min
- During this period, prepare the coating mix by diluting 10 mg/ml human fibronectin in Hank’s Balanced Salt solution (HBSS) to 0.1 mg/ml.
- When the coverslips are dry, pipette 100 µl of coating solution onto each glass coverslip so the solution covers only the glass without pooling around the outside of the well. Place the covered 24 well cluster plate in a cell culture incubator for at least an hour.
- Just before use, remove the coating solution. The same coating solution can be transferred to a sterile tube and used up to three times with no visible loss of efficacy.
2. Preparation of Endothelial Cell Monolayer
- Culture human coronary artery endothelial cells (EC) in medium at 37 °C and 5% carbon dioxide.
- Aspirate off culture media and rinse EC monolayer with 10 ml HBSS
- Aspirate off HBSS and add 2 ml 0.5% Trypsin, 0.2%EDTA solution. Incubate at RT for approximately 5 min.
- When EC can be dislodged by a tap to the side of the flask, add 5 ml of Soybean trypsin inhibitor and with pipette squirt the surface of the flask to further dislodge the cells.
- Transfer cells into a 15 ml tube and centrifuge the tube at 200 x g for 5 min.
- Aspirate off the supernatant and resuspend the EC pellet in 5 ml of media. Count the cells with a haemocytometer and adjust the cell concentration to 50,000 EC per ml of EC media.
- Dispense 1 ml of the cell suspension into each well of the 24 well plate containing coated glass coverslips. Incubate cells O/N in cell culture incubator at 37 °C plus 5% carbon dioxide.
- The cells are ready for use in experiments when a confluent monolayer is formed, within 3 days.
3. Preparation of Monocytes
- Transfer HL-60 cells that are cultured in RPMI supplemented with 10% fetal calf serum, as suspension culture, into a 15ml tube. Centrifuge the cells at 200 x g for 5 min.
- Remove the supernatant and resuspend cells in 5 ml media and adjust the cell concentration with media to 2 million cells/ml. If fluorescence labeling of the HL-60 is desired, resuspend the cells in RPMI without serum and proceed as described in section 4.
- Add 0.5 ml of HL-60 cell suspension into each well of the 24 well cluster plate containing EC monolayer and incubate the plate in a cell culture incubator at 37 °C and 5% carbon dioxide for a chosen fixed period of time between 1 and 3 hr. Little difference is observed between 1 hr and 3 hr time-point, where binding saturation is attained.
- Prepare for cell washing/harvesting: Fill a 120 ml tube with 100 ml of HBSS. Dispense 1 ml of formalin into each well of a fresh 24 well plate.
- After the incubation period, use a pair of sharp forceps and pick up the glass coverslip from the well and hold the coverslip vertical and dab the edge of the coverslip on a piece of tissue on the bench for 3 sec, taking care not to touch the cell-covered surface of the coverslip with the tissue.
- Holding the coverslip firmly with a pair of forceps, dunk the coverslip in and out of the HBSS five times.
- After the fifth dunk in HBSS, dab the edge of the coverslip on the tissue for 3 sec.
- Repeat the dunking and dabbing procedures described in 3.6 and 3.7, making a total of three sets of 5 dunks followed by a dab.
- Transfer the coverslip into a well containing 10% formalin to fix the cells at RT. The coverslip is ready for enumeration, for long-term storage or to be subjected to other procedures described below.
4. Labeling of HL-60 cells with Cell Tracker (optional)
- Count and transfer appropriate amount of HL-60 cells (e.g.,10 million) into a 15 ml centrifuge tube. Centrifuge HL-60 cells at 200 x g for 5 min. Remove supernatant.
- Resuspend cell pellet in Cell Tracker in RPMI media without serum at a concentration of 1 million cells/ml. Incubate cells in cell culture incubator for 1 hr.
- Centrifuge cells at 200 x g for 5 min and discard the supernatant. Resuspend cell pellet in media to a concentration of 2 million cells/ml.
- Add 0.5 ml of Cell Tracker-labeled HL-60 into each well containing endothelial cells grown on glass coverslip. Continue at 3.3.
5. Enumeration of Adhesive Endothelial Cells
- Mount coverslips on microscope slides with DAPI counter-stain to identify individual cells.
- Using an inverted microscope with a 4X or 10X objective, acquire images of several fields (e.g., 5 fields) and count the highest number of monocytes that aggregate on un-irradiated endothelial cells (This should typically be 3-5).
- Set twice this number as the threshold of monocytes on an endothelial cell to be that defined as a cluster. If required, a different criterion can be used for defining a cluster depending on the nature of the experiment.
- Count the number of clusters and the number of endothelial cells in the field. Divide the former by the latter to obtain the percentage of adhesive endothelial cells. Score numerous fields to obtain an average and standard deviation of the counts.
6. Counterstaining with Antibodies
- After the adhesion assay and cells on the coverslip have been fixed in 10% formalin for 15 min at RT, subject the coverslips to immunofluorescence using standard procedure10 and antibodies of choice.
- Add and remove the various solutions very gently to avoid dislodging monocytes that are attached to endothelial cells.
7. Counterstaining for Senescence-associated Beta-galactosidase Activity
- After the adhesion assay and cells on the coverslip have been fixed in formalin for 15 min, stain for senescence-associated beta-galactosidase activity as described in the instructions that accompany the staining kit. Add and remove the various solutions very gently to avoid dislodging monocytes that are attached to endothelial cells.
Representative Results
This method allows the detection of individual adhesive endothelial cells within a population. For example, while a monolayer of un-irradiated endothelial cells retained a few sporadic HL-60 monocytes following incubation and washing, endothelial monolayer at 7 days post-10Gy irradiation prior to incubation with monocytes were bound by monocytes in clusters around individual endothelial cells (Figure 1). Although this phenomenon is readily observable under phase contrast microscopy, fluorescence microscopy reveals an even clearer image of the monocyte clusters.
After performing the described adhesion assay, it is possible to proceed to staining the cells for membrane, cytoplasmic or nuclear (Figure 2) proteins using appropriate antibodies with standard immunofluorescence methods. Furthermore, enzyme-based assay such as senescence-associated beta-galactosidase (Figure 3) can also be performed.
Since each monocyte cluster corresponds to an individual endothelial cell, enumeration of the clusters will reveal the actual number of adhesive endothelial cells within the monolayer (Figure 4), and hence the percentage of adhesive endothelial cells within the population (Table 1, Figure 6). This is the only method to date that allows such quantification of endothelial cell adhesiveness. It is noteworthy that the endothelial cells used in the experiments here are contact-inhibited and do not increase in number after achieving confluence. This eliminates the potential complexity posed by increased endothelial cell number at later time points. If desired, monocytes attached to endothelial monolayers can be dislodged by 0.5% trypsin-0.2% EDTA solution (instead of fixation in 3.9) and their fluorescence measured using a plate reader, as is the case in contemporary methods (Figure 5).
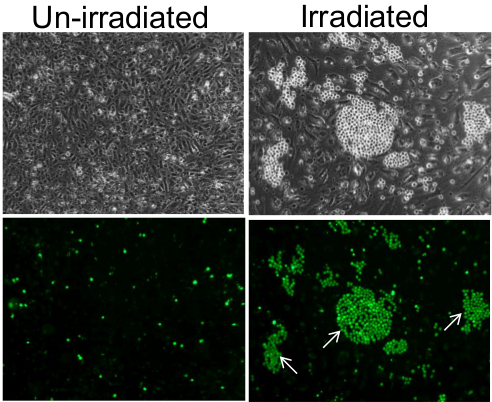
Figure 1. Adhesion of monocytes on monolayer of endothelial cells. On un-irradiated endothelial cells, monocytes adhered as individual cells in a sporadic and random manner (left panels) and in clusters on individual endothelial cells of the 7 days post-10 Gy irradiated monolayer (right panels). Lower panels are fluorescence images of the top panels, confirming that the small and bright spherical cells visible under phase contrast are indeed monocytes that were pre-labeled with Cell-Tracker Green. Some of the monocyte clusters are indicated by white arrows. Images were taken using a 10X objective. Please click here to view a larger version of this figure.
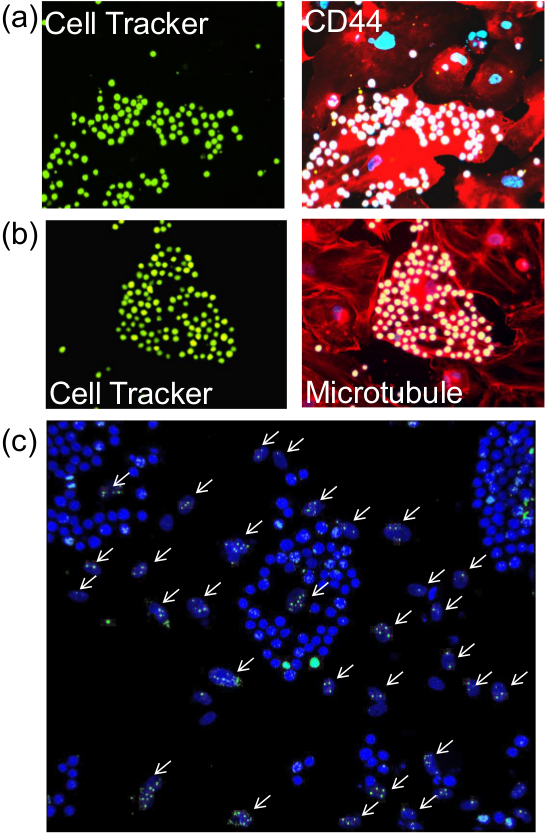
Figure 2. Staining of endothelial cells for proteins post-adhesion assay. After performing the described adhesion assay, cells on the glass coverslips were subjected to immunofluorescence for the detection of (A) membrane protein (CD44), b) cytoplasmic protein (Tubulin) or c) nuclear protein (γ-H2AX). Left panels of (A) and (B) (seen with 20X objective) show monocytes pre-labelled with Cell Tracker Green and the similar image on the right panels reveal CD44 and microtubules (red) and DAPI-stained nuclei (blue). White arrows in (C) point to irradiated endothelial cell nuclei that were stained with antibodies against γ-H2AX. Monocyte nuclei which are stained a brighter blue by DAPI are easily distinguished from the oval shaped nuclei of the endothelial cells. Images were taken with 20X objective. Please click here to view a larger version of this figure.
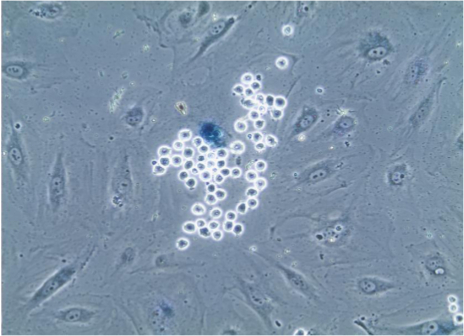
Figure 3. Senescence-associated beta-galactosidase staining of endothelial cells. After adhesion assay, cells on coverslips were subjected to staining for senescence-associated beta-galactosidase which causes lysosomes of senescent cells to turn blue, as is evident in the endothelial cell that is selectively bound by numerous monocytes, which are easily identified due to their small size and spherical shape. Image seen through 20X objective. Please click here to view a larger version of this figure.
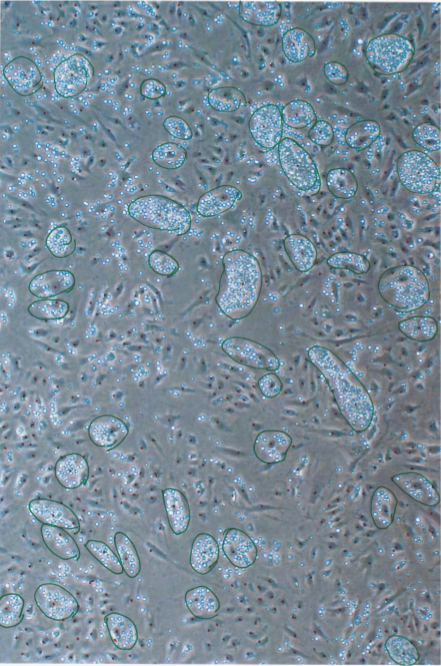
Figure 4. Enumeration of monocyte clusters on individual endothelial cells. After adhesion assay, images of the cells were taken from several different positions and monocyte clusters identified and circled (in green). A cluster was defined as an agglomeration of 10 or more monocytes on an endothelial cell. The number of monocyte clusters and number of 12 days post-irradiated endothelial cells (Dotted in red) in the image were counted and percentage of adherent endothelial cells calculated as shown in Table 1. Image taken through 10X objective. Please click here to view a larger version of this figure.
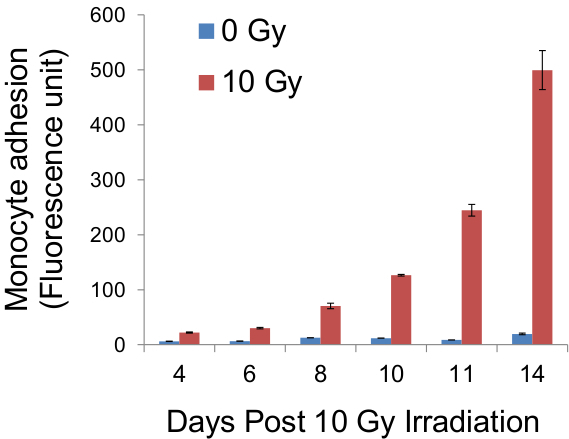
Figure 5. Quantification of fluorescence of adherent monocytes. Following adhesion assay, cells on glass coverslips were trypsinised and the fluorescence of monocytes pre-labelled with CellTracker Green was measured, using a fluorescence plate reader, as an indirect indicator of the adhesiveness of the endothelial monolayer. Results from such measurements at stated days post-irradiation were obtained and platted on the graph above.
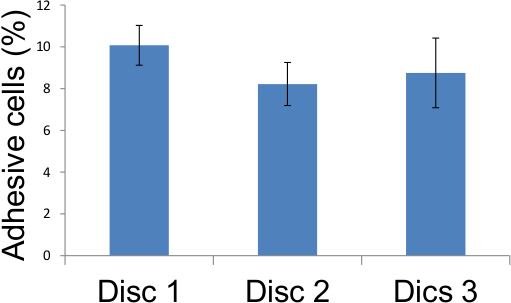
Figure 6. Representation of adhesion assay scores from Table 1. Adhesive endothelial cells in five different locations on three glass coverslips were scored as described in Figure 4 and the results tabulated above demonstrating that 12 days after 10 Gy irradiation, 8 to 10 percent of irradiated endothelial cells became adhesive.
| Percentage of adhesive endothelial cells |
|||
| Disc 1 | Disc 2 | Disc 3 | |
| Field 1 | 8.65 | 8.29 | 8.21 |
| Field 2 | 10.44 | 7.27 | 7.21 |
| Field 3 | 9.63 | 9.05 | 8.33 |
| Field 4 | 11.11 | 7.09 | 8.41 |
| Field 5 | 10.55 | 9.4 | 11.61 |
| Average | 10.07 | 8.22 | 8.75 |
Table 1. Adhesion assay scores. Adhesive endothelial cells in five different locations on three glass coverslips were scored as described in Figure 4 and the results tabulated above demonstrating that 12 days after 10 Gy irradiation, 8 to 10 percent of irradiated endothelial cells became adhesive.
Discussion
The monocyte adhesion assay described above was used successfully in experiments designed to study the biological effects of ionizing radiation on endothelial monolayers10. Although this is not the only method available to assess the adhesiveness of an endothelial monolayer, it is the only method that allows the quantification of the proportion or percentage of endothelial cells within a monolayer that is adhesive. This is an important distinction as a global quantitative change in adhesion of monocytes, as measured by other methods can be attributed either to a general rise in adhesiveness of all cells of the endothelial monolayer or to the increase adhesiveness of a sub-population of endothelial cells within the monolayer, as shown in the example above. The value of having this information is exemplified by the fact that the ability to quantify the percentage of endothelial cells that exhibited enhanced adhesiveness after irradiation led to the inescapable conclusion that this effect is not due to genetic mutation of a particular gene as the percentage of cells that were adhesive were greatly above (over 1,000 times more) that which would be expected from random mutation of a gene by X-radiation at that dose.
The factor that allows good reproducibility of the results is the washing regime. As the washing procedure involves the immersion of the glass coverslip into the wash buffer followed by dabbing on a tissue, variability in the buffer turbulence which inevitably arises from the old method of pipetting of the wash buffer into a well is avoided. Indeed it was the observation of excessive variability between replicates obtained using the pipetting method that compelled us to devise a washing procedure that removed the variability element from the washing step.
Admittedly, the limitations of this assay lie in the need to carry out manual count of monocyte clusters, which do not allow it to be adapted for high throughput analyses. Secondly, the decision on how many monocytes constitute a cluster has to be determined semi-arbitrarily and the level may be set too high and result in the exclusion of some genuine clusters. The lack of shear force in this method can also be viewed as a limitation in experiments in which grossly enhanced adhesiveness of endothelial cells is induced (e.g., +TNFα). In other situations however, the lack of shear force is an advantage as it allows the detection of less exaggerated augmentation of adhesiveness. Modest increase in monocyte adhesiveness can be particularly important and relevant. In vivo, monocytes would not (in a typical experimental time) be expected to attach in great numbers to endothelial cells with modest increase in adhesiveness, largely due to shear force. In time however, some monocytes probably would, and this is more likely to represent the environment of chronic inflammation. As such, the absence of shear force can be an advantage as it provides the opportunity for small increases in endothelial cell adhesiveness to be revealed in experimental time frames which are typically short and possibly too fleeting to allow modest increase in adhesiveness to be observed under shear stress.
The ability to subject the cells after this assay to other analyses such as senescence assay and immunofluorescence staining increases the usefulness of this method as it allows the association of adhesiveness of specific endothelial cells to particular cellular proteins or cellular states as demonstrated above, a feature that is not available with the older adhesion assays.
This method has been used with est2-immortalised human coronary endothelial cells and similar results were also obtained with primary human coronary endothelial cells10, demonstrating that it is not specific to just a particular cell line. Also, the adoption of this method of assaying adhesiveness of endothelial cells do not preclude subjecting the same cells to the standard adhesion assay that measures fluorescence of labeled immunocytes. Together, this report demonstrates that this method is versatile, inclusive and provides much more information than the standard adhesion assay.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We are very grateful to Simon Bouffler for his full support and to Public Health England for infrastructure support. This work was supported by Public Health England throughthe National Institute for Health Research (NIHR) grant. This report is work commissioned by the NIHR. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Materials
| Hepes Buffered Saline Solution (HBSS) | Sigma | H6648 | |
| Glass coverslips Round 12mm Diameter | Menzel-Glaser | CB00120RA1 | |
| 24-well cluster plate | Costar | 3526 | |
| Meso-Endo Cell media | Cell Applications | 212-500 | |
| Trypsin-EDTA | Sigma | T4174 | |
| Soybean trypsin inhibitor | Life Technologies | 17075-029 | |
| Cell Tracker Green | Life Technologies | C7025 | |
| RPMI | Sigma | R8758 | |
| Foetal Calf Serum | Life Technologies | 10500064 | |
| Beta glasctosidase Assay kit | CellSignaling | 9860 | |
| Fibronectin | Sigma | F0895 |
References
- Ortega-Gomez, A., Perretti, M., Soehnlein, O. Resolution of inflammation: an integrated view. EMBO Mol Med. 5 (5), 661-674 (2013).
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 83 (2), 456S-460S (2006).
- Ikuta, S., Kirby, J. A., Shenton, B. K., Givan, A. L., Lennard, T. W. Human endothelial cells: effect of TNF-alpha on peripheral blood mononuclear cell adhesion. Immunology. 73 (1), 71-76 (1991).
- Watson, C., et al. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin Exp Immunol. 105 (1), 112-119 (1996).
- Sans, M., et al. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterology. 116 (4), 874-883 (1999).
- Su, Y., Lei, X., Wu, L., Liu, L. The role of endothelial cell adhesion molecules P-selectin, E-selectin and intercellular adhesion molecule-1 in leucocyte recruitment induced by exogenous methylglyoxal. Immunology. 137 (1), 65-79 (2012).
- Hallahan, D., Kuchibhotla, J., Wyble, C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 56 (22), 5150-5155 (1996).
- Vaporciyan, A. A., Jones, M. L., Ward, P. A. Rapid analysis of leukocyte-endothelial adhesion. J Immunol Methods. 159 (1-2), 93-100 (1993).
- Prabhakarpandian, B., Shen, M. C., Pant, K., Kiani, M. F. Microfluidic devices for modeling cell-cell and particle-cell interactions in the microvasculature. Microvasc Res. 82, 210-220 (2011).
- Lowe, D., Raj, K. Premature aging induced by radiation exhibits pro-atherosclerotic effects mediated by epigenetic activation of CD44 expression. Aging Cell. 13 (5), 900-910 (2014).

