Synthesis and Characterization of High c-axis ZnO Thin Film by Plasma Enhanced Chemical Vapor Deposition System and its UV Photodetector Application
Summary
We offered a method to directly synthesize high c-axis (0002) ZnO thin film by plasma enhanced chemical vapor deposition. The as-synthesized ZnO thin film combined with Pt interdigitated electrode was used as sensing layer for ultraviolet photodetector, showing a high performance through a combination of its good responsivity and reliability.
Abstract
In this study, zinc oxide (ZnO) thin films with high c-axis (0002) preferential orientation have been successfully and effectively synthesized onto silicon (Si) substrates via different synthesized temperatures by using plasma enhanced chemical vapor deposition (PECVD) system. The effects of different synthesized temperatures on the crystal structure, surface morphologies and optical properties have been investigated. The X-ray diffraction (XRD) patterns indicated that the intensity of (0002) diffraction peak became stronger with increasing synthesized temperature until 400 oC. The diffraction intensity of (0002) peak gradually became weaker accompanying with appearance of (10-10) diffraction peak as the synthesized temperature up to excess of 400 oC. The RT photoluminescence (PL) spectra exhibited a strong near-band-edge (NBE) emission observed at around 375 nm and a negligible deep-level (DL) emission located at around 575 nm under high c-axis ZnO thin films. Field emission scanning electron microscopy (FE-SEM) images revealed the homogeneous surface and with small grain size distribution. The ZnO thin films have also been synthesized onto glass substrates under the same parameters for measuring the transmittance.
For the purpose of ultraviolet (UV) photodetector application, the interdigitated platinum (Pt) thin film (thickness ~100 nm) fabricated via conventional optical lithography process and radio frequency (RF) magnetron sputtering. In order to reach Ohmic contact, the device was annealed in argon circumstances at 450 oC by rapid thermal annealing (RTA) system for 10 min. After the systematic measurements, the current-voltage (I–V) curve of photo and dark current and time-dependent photocurrent response results exhibited a good responsivity and reliability, indicating that the high c-axis ZnO thin film is a suitable sensing layer for UV photodetector application.
Introduction
ZnO is a promising wide-band-gap functional semiconductor material due to its unique properties such as high chemical stability, low cost, non-toxicity, low power threshold for optical pumping, wide direct band gap (3.37 eV) at RT and large exciton binding energy of ~60 meV 1-2. Recently, ZnO thin films have been employed in many application fields including transparent conductive oxide (TCO) films, blue light emitting device, field-effect transistors, and gas sensor 3-6. On the other hand, ZnO is a candidate material to replace indium tin oxide (ITO) owing to indium and tin being rare and expensive. Moreover, ZnO possesses high optical transmittance in the visible wavelength region and low resistivity compared with ITO films 7-8. Accordingly, fabrication, characterization and application of ZnO has been extensively reported. This present study focuses on synthesizing high c-axis (0002) ZnO thin films by a simple and effectively method and its practical application towards a UV photodetector.
The recent research report findings indicate that the high quality ZnO thin film could be synthesized by various techniques such as sol-gel method, radio frequency magnetron sputtering, metal organic chemical vapor deposition (MOCVD), and so on 9-14. Each technique has its advantages and disadvantages. For example, a principal advantage of sputtering deposition is that target materials with very high melting point are effortlessly sputtered onto the substrate. In contrast, the sputtering process is difficult to combine with a lift-off for structuring the film. In our study, the plasma enhanced chemical vapor deposition (PECVD) system was employed to synthesize high quality c-axis ZnO thin films. Plasma bombardment is a key factor in the synthesizing process that can increase the thin film density and enhance the ion decomposition reaction rate 15. In addition, the high growth rate and large-area uniform deposition are other distinctive advantages for PECVD technique.
Except for the synthesis technique, the good adhesion on the substrate is another considered issue. In many studies, the c-plane sapphire has been widely used as the substrate to synthesize high c-axis ZnO thin films because the ZnO and sapphire have the same hexagonal lattice structure. However, the ZnO was synthesized on sapphire substrate exhibiting rough surface morphology and high residual (defect-related) carrier concentrations due to the large lattice misfits between the ZnO and c-plane sapphire (18%) oriented in the in-plane direction 16. Compared with the sapphire substrate, a Si wafer is another widely used substrate for the ZnO synthesis. Si wafers have been extensively used in semiconductor industry; and thus, growth of high quality ZnO thin films on Si substrates is very important and necessary. Unfortunately, the crystal structure and thermal expansion coefficient between the ZnO and Si are obviously different leading to deterioration of crystal quality. Over past decade, great efforts have been made to improve the quality of ZnO thin films onto Si substrates by using various methods including ZnO buffer layers 17, annealing in various gas atmosphere 18, and passivation of the Si substrate surface 19. The present study successfully offered a simple and effectively method to synthesize high c-axis ZnO thin film onto Si substrates without any buffer layer or pre-treatment. The experiment results indicated that the ZnO thin films synthesized under the optimal growth temperature showed the good crystal and optical qualities. The crystalline structure, RF plasma composition, surface morphology, and optical properties of ZnO thin films were investigated by X-ray diffraction (XRD), optical emission spectroscopy (OES), field emission scanning electron microscopy (FE-SEM), and RT photoluminescence (PL) spectra, respectively. Moreover, the transmittance of ZnO thin films was also confirmed and reported.
The as-synthesized ZnO thin film served as a sensing layer for UV photodetector application was also investigated in this study. The UV photodetector has great potential applications in UV monitoring, optical switch, flame alarm, and missile warming system 20-21. There are many types of photodetectors which have been carried out such as positive intrinsic negative (p-i-n) mode and metal-semiconductor-metal (MSM) structures including Ohmic contact and Schottky contact. Each type has its own advantages and drawbacks. Currently, MSM photodetector structures have attracted intensive interest due to their outstanding performance in responsivity, reliability and response and recovery time 22-24. The results presented here have shown that the MSM Ohmic contact mode was employed to fabricate ZnO thin film based UV photodetector. Such a kind of photodetector typically reveals a good responsivity and reliability, indicating that the high c-axis ZnO thin film is a suitable sensing layer for UV photodetector.
Protocol
1. Substrate Preparation and Cleaning
- Cut 10 mm x 10 mm silicon substrates from Si(100) wafer.
- Cut 10 mm x 10 mm glass substrates.
- Use ultrasonic cleaner to clean the silicon and glass substrates with acetone for 10 min, alcohol for 10 min, and then isopropanol for 15 min.
- Rinse the substrates with deionized (DI) water three times.
- Blow-dry the substrates with a nitrogen gun.
2. DEZn Preparation and Preservation
Note: Diethylzinc (C2H5)2Zn, also called DEZn, is a highly pyrophoric organozinc compound consisting of a zinc center bound to two ethyl groups. Never work alone when using DEZn. DEZn is very toxic and sensitive to the oxygen and water, be sure not to place the DEZn near the water. Always wear protective masks and eye protection; all procedures must be performed in the hood. Most importantly, unused DEZn must be stored in a 5 oC environment.
Note: For the first use of DEZn, follow step 2. If not, start the experiment from step 3.
- Use syringe to draw out 30 ml DEZn from the bottle and then inject into a beaker placed in a steel cylinder.
- Use a galvanized iron pipe to connect the steel cylinder with the reaction chamber.
- Use mechanical pump and ball valve to pump down the steel cylinder in vacuum environment (to 6 Torr).
Note: DEZn will severely react with oxygen, it must be maintained in the vacuum environment. - Store the unused DEZn in a 5 oC environment.
3. PECVD Chamber Preparation and Synthesis of ZnO Thin Films
Note: The schematic diagram of plasma enhanced chemical vapor deposition is depicted in Figure 1.
- Set the working distance between showerhead electrode and sample stage at 30 mm.
- Place the substrates on the sample stage of reaction chamber in proper location where there is a 3 cm distance from the DEZn inlet.
- Open the rotary pump and gradually open the gate valves and butterfly valve.
- Wait until the background pressure of the reactor chamber is lower than 30 mTorr.
- Close the gate valves and butterfly valve, which connects to the rotary pump.
- Then open the turbo pump and relative gate valves to reach high vacuum of 3 x 10-6 Torr.
- After reaching the necessary vacuum condition, open the heat controller and heat the sample stage to the synthesis temperature (200, 300, 400, 500, and 600 oC for different experiment parameters).
- When the temperature and the pressure reach the necessary condition, close the turbo pump and then open the gate valves and butterfly valve which connects to the rotary pump simultaneously.
- Next, open the gas inlet valves and turn on the argon gas flow controller simultaneously.
- Flow the argon gas (0.167 ml/sec) into the chamber.
- Set the chamber pressure to 500 mTorr.
- Turn on the RF (13.56 MHz) generator and matching network, then set the RF power at 100 W for purging the samples surface for 15 min.
- After finishing the purge of samples, turn the RF power down to 70 W.
- Next, turn on the carbon dioxide gas controller and gas inlet valve.
- Flow the carbon dioxide (0.5 ml/sec) into the chamber.
- Set the working pressure at 6 Torr.
- After the chamber pressure reaches 6 Torr, flow the high pure argon as carrier gas (0.167 ml/sec) for carrying diethylzinc (DEZn) into the chamber and open ball valve connected to the DEZn simultaneously. At the same time, start the synthesis of ZnO films.
- Continue the plasma synthesis of ZnO films for 5 min.
- After the ZnO films have been synthesized, seriatim turn off the RF generator, ball valve, heat controller and all of gas flow controllers along with gas inlet valves.
- Take out the sample when the sample stage temperature cools down to RT. Note: The cooling rate is approximately 1.8 oC/min.
4. Preparation of Interdigitated-like Pattern onto As-synthesized ZnO Thin Film
Note: The schematic of lithography process is depicted in Figure 3.
- Use a hot plate to bake the as-synthesized ZnO sample at 150 oC for 10 min.
- Place the sample on the spin coater, and then dispense the liquid solution of photoresist (S1813) with 100 µl onto the ZnO sample.
- Run the spin coater at 800 rpm for 10 sec and then accelerate to 3,000 rpm for 30 sec to produce a uniformly thin layer.
- Soft bake the photoresist-coated ZnO sample at 105 oC for 90 sec.
- After the soft-baking, use UV light to expose the photoresist-coated sample trough a photomask by mask aligner. The exposure time is 2 sec and the power is 400 W.
Note: The pattern of photomask is designed as interdigitated-like, which is 0.03 mm wide and 4 mm long (14 pairs) and has an inter-electrode spacing of 0.15 mm as depicted in Figure 2. It is worth noting that the total photosensitive area is 84.32 mm2 for the detector. - After the exposure procedure, use tweezers to clip the sample, and then immerse into the diluted developer (mix 50 ml of developer and 150 ml of deionized water) through actions of swinging from side to side for 35 s to obtain the developed sample.
- Rinse the developed sample with DI water and dry with nitrogen gas.
- Use the optical microscope to check the pattern intact. If not, use acetone to remove the photoresist and repeat steps 4.2 to 4.7 until the perfect pattern has been obtained.
- Hard bake the sample at 120 oC for 20 min.
5. Deposition of Pt Top Electrode and Chemical Lift-off
- Use the RF magnetron sputtering system to deposit a thin conductive Pt layer (100 nm) on the top of the developed sample before proceeding to chemical lift-off procedure.
- Set the distance between target and substrate at 13 mm.
- Use the mechanical pump to reach a rough vacuum of 5 mTorr.
- Then, use the turbo pump to obtain a high vacuum of 7 x 10-7 Torr.
- Wait until the chamber reaches the high vacuum, close the turbo pump and open the mechanical pump subsequently.
- Flow the argon gas at 0.3 ml/sec into the chamber by mas flow controller until the chamber pressure reach the working pressure of 100 mTorr.
- Turn on the direct current (DC) discharge power supply and set the DC power at 15 W for sputtering the Pt thin film electrode onto the sample for 25 min.
- After the Pt electrode layer has been deposited by magnetron sputtering method, take out the sample from the chamber.
- Immerse the sample into the acetone liquid for chemical lift-off process by ultrasonic cleaner to remove the photoresist.
- Set the cleaning time at 1 min to thoroughly remove photoresist, and then obtain the interdigitated-like Pt electrode onto the ZnO thin film.
6. RTA process
- Place the as-fabricated Pt/ZnO sample into the RTA system.
- Use the mechanical pump and gate valve to pump down the RTA chamber pressure to 20 mTorr.
- Wait until the chamber pressure reaches 20 mTorr, flow argon gas at 0.3 ml/sec into the chamber and set the working pressure of 5 Torr.
- Next, set the heating rate as 100 oC/min.
- Then, anneal the sample at 450 oC for 10 min.
- Once annealed, wait until the sample cools to RT, then take out the sample.
Representative Results
The ZnO (0002) thin films with high c-axis preferred orientation have been successfully synthesized onto the Si substrates by using the PECVD system. The carbon dioxide (CO2) and the diethylzinc (DEZn) were used as oxygen and zinc precursors, respectively. The crystal structure of ZnO thin films was characterized by X-ray diffraction (Figure 4), indicating that the ZnO thin film synthesized at 400 oC with the strongest (0002) diffraction peak. When the synthesized temperature increased up to 500 oC, the (0002) diffraction peak became weaker accompanying with appearance of (10-10) diffraction peak. Particularly, all ZnO diffraction peaks will disappear when the synthesized temperature is set at 600 oC. The in–situ OES was employed to monitor the plasma chemical composition during the ZnO synthesized process (Figure 5). The results indicate that Zn, O2 and carbon monoxide (CO) emission peaks were detected when the synthesis of ZnO was occurring. The FE-SEM images reveal that the ZnO thin films show different surface morphologies with different synthesized temperatures (Figure 6A-E). The homogeneous surface and with small grain size distribution were obtained at 300 and 400 oC. The optical properties of ZnO thin films were determined by PL spectra (Figure 7). The results indicate that the ZnO thin films synthesized at 300 and 400 oC showing a strong NBE emission and with a negligible DL emission. In addition, the NBE emission shifts to short wavelength with increasing the temperature from 300 to 600 oC. The transmittance measurement indicates that the ZnO thin films synthesized at 200, 300 and 400 oC have good transparency with an average visible transmittance higher than 80% (Figure 8A-B). Interestingly, the transmittance declined dramatically when the synthesized temperatures were increased up to excess of 500 oC.
The performance of UV photodetector combined with ZnO thin film and Pt interdigitated electrode has been investigated. From the characteristics, the current of the photodetector was obviously enhanced under light illumination, compared with that under dark conditions (Figure 9). It was also be observed that the I–V curves are symmetric, reflecting a MSM Ohmic contact behavior between the ZnO thin film and the Pt electrode. The time-dependent photo-current response of the photodetector was measured with switching off and turning on the UV light (38 mW/cm2) five times at the bias of 5V (Figure 10).
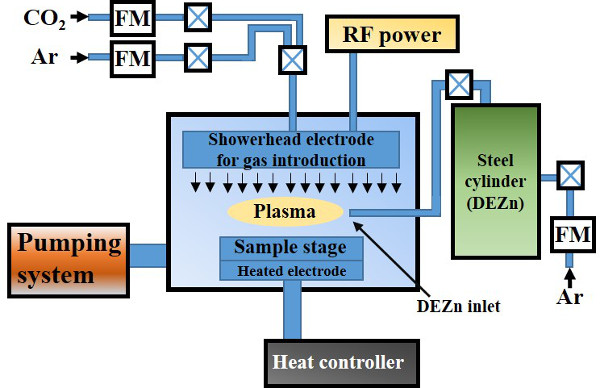
Figure 1. Schematic diagram of the plasma enhanced chemical vapor deposition system. Please click here to view a larger version of this figure.
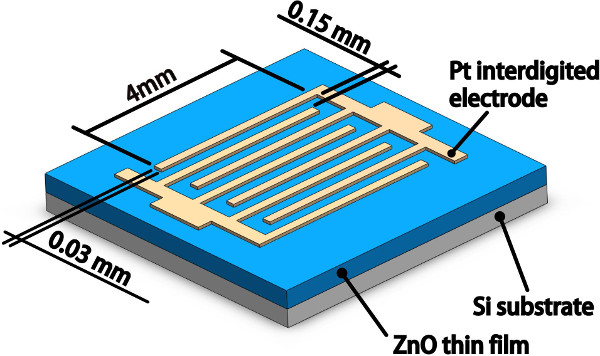
Figure 2. Schematic diagram of the ZnO based UV photodetector combined with Pt interdigitated electrode. Please click here to view a larger version of this figure.
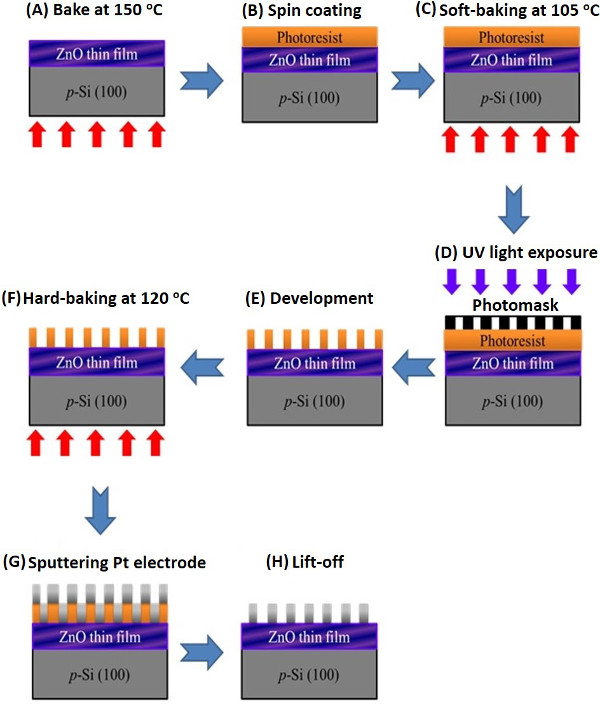
Figure 3. Flow chart of the lithographic process for Pt interdigitated electrode fabricated onto the ZnO films synthesized on silicon substrates. Step (A) uses the hot plate to bake the as-synthesized ZnO thin film at 150 oC for 10 min to remove the surface moisture. Step (B) spin coats the photoresist onto ZnO thin film. Step (C) soft bakes the photoresist-coated ZnO sample at 105 oC for 90 s to remove excess photoresist solvent. Step (D) exposes UV light through a photomask for 2s. Step (E) uses the developer to remove the photoresist. Step (F) hard bakes the sample at 120 oC for 20 min to make a more durable protecting layer for preparing the next RF magnetron sputtering. Step (G) uses the RF magnetron sputtering to deposit a thin Pt layer onto the developed sample. Step (H) uses the acetone to lift-off the sample. Please click here to view a larger version of this figure.
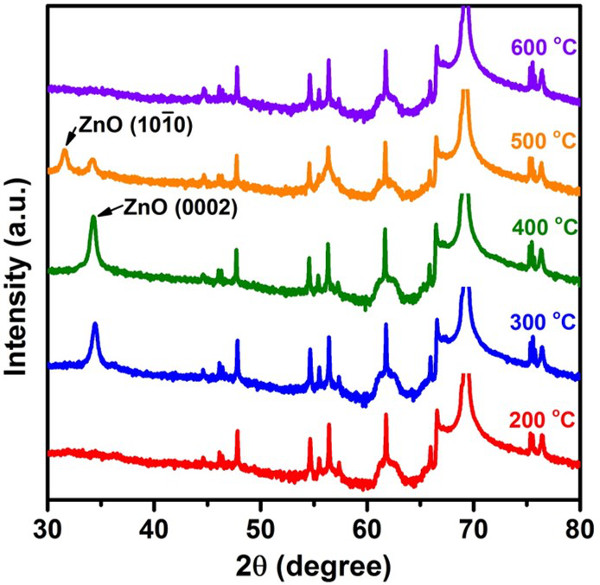
Figure 4. X-ray diffraction patterns for the ZnO thin films synthesized at different synthesis temperatures ranged from 200 to 600 oC. X-ray was emitted by Cu Kα radiation (λ = 1.54 Å). The scan angle was set from 30o to 80o, step size was 0.01o and time per step was 0.15 sec. The ZnO (0002) diffraction peak is located at 34.24o, whereas the ZnO (10-10) diffraction peak is located at 31.59o. The others diffraction peaks come from substrate signals. Please click here to view a larger version of this figure.
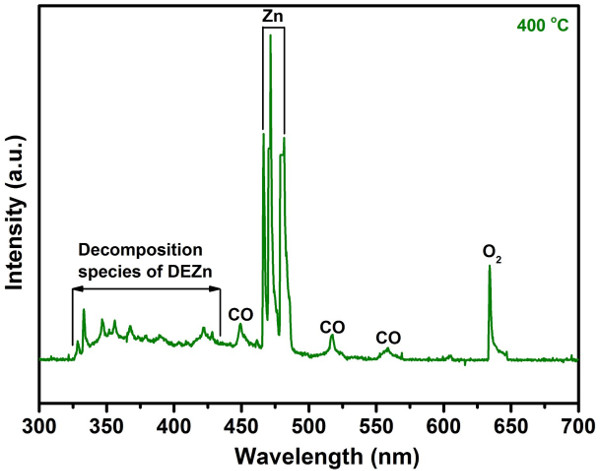
Figure 5. In–situ OES spectrum for the RF plasma during ZnO thin film synthesized at 400 oC. The data was confirmed from reference 28. The peaks signals located at 449, 517 and 559 nm are determined as CO species. The peaks signals located at 466, 471 and 482 nm are determined as Zn species, and 634 nm is determined as O2 species. The complicated signals located in the range between 325 and 430 nm are determined as decomposition species of DEZn. The monitored time was 7 sec. Please click here to view a larger version of this figure.
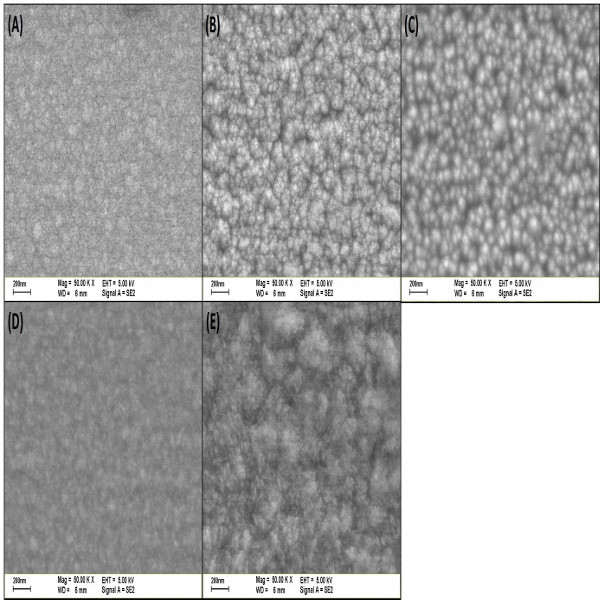
Figure 6. Plane-view SEM images for the ZnO thin films synthesized at different synthesis temperatures, including (A) 200 (B) 300 (C) 400 (D) 500, and (E) 600 oC, respectively. Please click here to view a larger version of this figure.
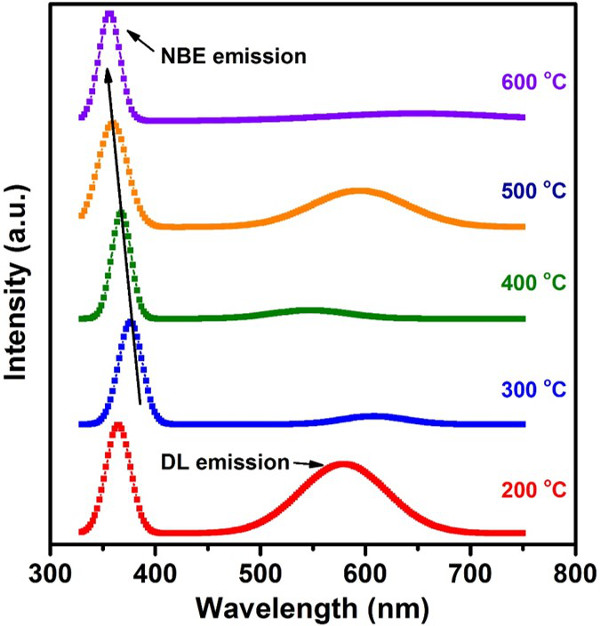
Figure 7. RT PL spectra for the ZnO thin films synthesized at different synthesis temperatures ranged from 200 to 600 oC. The PL measurement used 325 nm He-Cd laser under 100% laser power efficiency. The exposure time was 10 sec. The detection range was from 325 to 750 nm. Please click here to view a larger version of this figure.
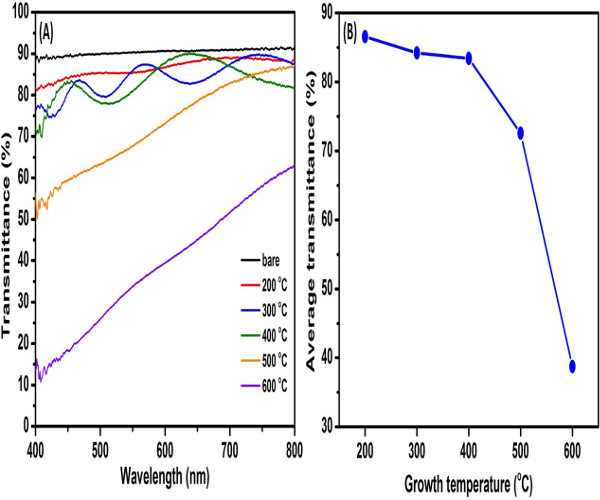
Figure 8. Optical transmittance spectra for the ZnO thin films synthesized at different synthesis temperatures.(A) Typical optical transmittance spectra measured from 400 to 800 nm; (B) average transmittance values as a function of the synthesis temperature.Please click here to view a larger version of this figure.
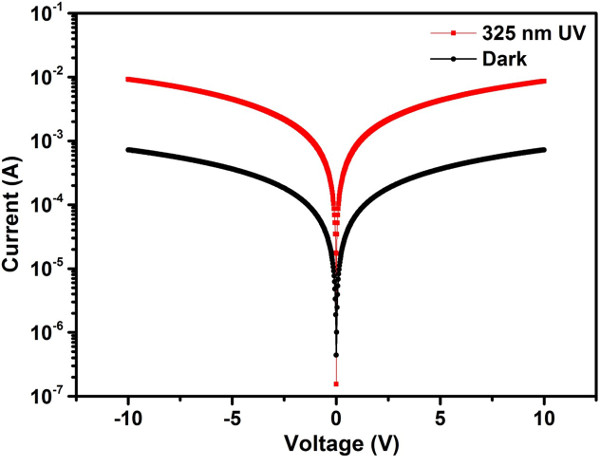
Figure 9. I–V characteristics of the fabricated ZnO thin film based UV photodetector. The measurement was under 325 nm UV light illumination with power density of 38 mW/cm2 (red curve) and the dark condition (black curve), and the testes were at bias voltage from -10 V to 10 V. Please click here to view a larger version of this figure.
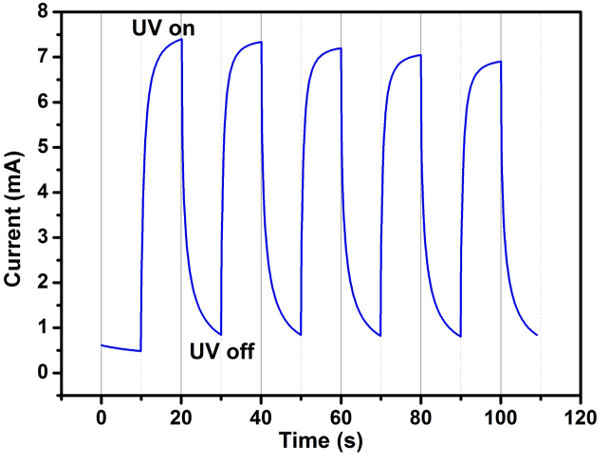
Figure 10. Time-dependent photocurrent response for the ZnO thin film based UV photodetector. The measurement was implemented over 5 times turning switching on/off circles at bias of 5 V under UV light (38 mW/cm2), in which both turn-on and turn-off time of the UV light were 10 sec. Please click here to view a larger version of this figure.
Discussion
Critical steps and modifications
In step 1, the substrates should be thoroughly cleaned and steps 1.3 to 1.5 followed to make sure that there is no grease or organic and inorganic contaminations on the substrates. Any grease or organic and inorganic contaminations on the substrate surface will significantly reduce the adhesion of the film.
Step 2 is the most important procedure before the ZnO film preparation process. DEZn is very toxic and violently reacts with water and easily ignites upon contact with air. DEZn must be very carefully injected into the steel cylinder and then pumped down to 6 Torr immediately. In step 3, make sure that each experimental parameter and step is accurately completed because even slightly different settings will lead to different results.
Step 4 must be carried out in a lithography laboratory and a clean room to avoid non-UV light illumination and dust-pollutionor particle-pollution effects. In step 5, power of the DC discharge power supply should be raised slowly; otherwise the Pt target will be broken. In step 6, the samples should be placed in the middle of the RTA chamber to ensure that the sample can be uniformly heated.
Data Analysis
The synthesized temperature is a crucial parameter for synthesizing high quality c-axis ZnO thin film because the thermal expansion coefficients and thermal mismatch strain effect with interfacial energy between the ZnO thin films and the Si substrate can significantly affect the crystallographic orientation. It can be clearly observed from XRD patterns (Figure 4). The strongest ZnO (0002) diffraction peak was obtained at 400 oC, indicating that the temperature is an optimal synthesized temperature for synthesizing c-axis ZnO thin film onto Si substrate by PECVD method. With a further increase in synthesized temperature up to 500 oC, leading to the deterioration of ZnO crystal quality. Furthermore, the ZnO thin film converted to amorphous phase when the synthesized temperature was set at 600 oC. It can be assumed that the high enough temperature (i.e., 400 oC) can provide sufficient energy, enhancing the ability of molecules to find the stable site and resulting in the ZnO thin film with high crystal quality. Simultaneously, the common defects including vacancies, interstitials, and dislocations in ZnO are also reduced. However, the ZnO will decompose into Zn and O2 molecules when excessive high temperature (i.e., 500 and 600 oC) was employed 25. Moreover, the different thermal expansion coefficients between ZnO (6.7 x 10-6 K-1) 26 thin film and Si (4.18 x 10-6 K-1) 27 substrate at 900 K will yield the thermal mismatch strain effect with interfacial energy. The above phenomena could deteriorate the crystal quality of ZnO thin film, resulting in the polycrystalline or amorphous phase.
For monitoring the plasma chemical composition during the ZnO synthesized process, the in–situ OES spectrum analysis of the RF plasma was carried (Figure 5). The OES analysis result illustrated that the three strong Zn emission peaks appeared around 475 nm and other distinct emission peaks were determined as O2 and CO signals 28. Moreover, some complicated emission peaks located in the range between 325 and 430 nm are determined as decomposition species of DEZn. The above beneficial information indicates that the OES is a useful instrument for in–situ monitoring of plasma chemical composition during the synthesized process.
The surface morphologies of the ZnO thin films were observed by FE-SEM (Figure 6A-E). It can be seen that the ZnO synthesized at 300 and 400 oC show densely packed with spherical grains and with small grain size distribution. Compared with that synthesized condition, other ZnO thin films exhibit disorderly and irregular surface, implying the polycrystalline or amorphous phase. It is worth noting that the SEM images are consistent with the previous XRD results.
The optical properties were determined by RT PL spectrum with 325 nm He-Cd laser. From the PL measurement (Figure 7), all ZnO thin films show a strong NBE emission peak located at around 375 nm, which is attributed to recombination of free-excitons through an exciton-exciton collision process 29. Simultaneously, the broad defect-related DL emission peaks located at around 575 nm are obtained when the synthesized temperatures were set at 200 and 500 oC. It is also found that the NBE emission has a blue-shift to shorter wavelengths with increasing synthesized temperature. In general, the DL emission is referred to the impurities and various structural defects in the ZnO phase such as oxygen vacancy and zinc interstitial 30, and the blueshift is due to the strong residual anisotropic strain in the ZnO thin film 31. Therefore, the PL results reflect that the synthesized temperature can significantly affect the optical properties of ZnO thin films. Among all ZnO samples, only two samples which were synthesized at 300 and 400 oC show a dominated NBE emission peak and negligible DL emission accompanying the high (0002) XRD diffraction peak.
The transparency for all synthesized ZnO thin films were measured by transmittance spectrum (Figure 8A) and the average transmittance value for each ZnO sample has been calculated (Figure 8B). The average transmittance value of ZnO samples synthesized at 200, 300 and 400 oC in the entire visible region is about 80%, but the samples synthesized at 500 and 600 oC show a relatively low transmittance, especially for the ZnO sample synthesized at 600 oC. The reason for the reduced transmittance of ZnO sample synthesized at higher temperature is still unclear until now. However, the high c-axis preferential orientation ZnO with high transparency has been obtained, indicating that the character could be used for future high-transmittance ZnO based optoelectronic devices.
Because the ZnO phase can adsorb oxygen molecules in the general environment and desorb oxygen molecules under UV illumination, it can be used as UV photodetector. Oxygen molecules can adsorb onto a ZnO surface by capturing free electrons from the conduction band of the ZnO [O2 +e–→ O2–], which yields a depletion layer near the surface, leading to greatly decrease the conductivity of the ZnO. When ZnO is illuminated by photon energy more than band gap of the ZnO (i.e., UV light), electron-hole pairs will be generated [hʋ→ e– + h+]. These carriers from electron-hole pairs will move toward the surface and neutralize the adsorbed oxygen molecules [h+ + O2–→ O2]. The neutralization oxygen molecules can effortlessly desorb form the surface of ZnO, which makes the conductivity increase significantly. Therefore, according to this theory, the high c-axis ZnO thin film was used as a sensing layer to fabricate UV photodetector. The UV detection characteristics of the fabricated Pt/ZnO photodetector were investigated and confirmed by measuring the I–V curve with and without UV light illumination and time-dependent photocurrent response (Figure 9 and 10). An obvious symmetric behavior could be observed, indicating that the high quality Ohmic contact has been achieved at the Pt/ZnO interface after post-annealed in argon at 450 oC by RTA. According to the measured I–V curves, the dark current is about 0.36 mA and the photo-current is about 4.3 mA at 5 V, indicating the current difference between the UV illuminated and dark condition. Because the performance of UV photodetector is critical dependent on its response and recovery times, the time-dependent photo-current response was implemented. The response time is usually defined as the time to approach 90 % of the maximum photo-current, and recovery time is the time to decay to 10 % of the maximum photo-current. From the time-dependent photo-current response measurement, the response and recovery time are about 3 and 9 s, respectively. Moreover, the UV light was switched on and off five times for testing the reliability. According to the above measurements, the ZnO based UV photodetector shows a fast responsivity and high reliability, which could be used in potential development for commercial UV photodetector application.
In summary, this present study provides a method to synthesize high c-axis preferential orientation ZnO thin film onto Si substrates by PECVD with a fine control of the synthesized temperature. The optimal c-axis ZnO thin film was synthesized at 400 oC showing a competitive and functional characteristic in terms of crystal structure, optical property, and transparency to the visible light. The ZnO based UV photodetector combined with Pt interdigitated electrode with Ohmic contact exhibits a fast responsivity and high reliability under UV light (38 mW cm-2) at the bias of 5 V. This present work could be a valuable direction and application both in research and industry.
Potential Benefits and Drawbacks of PECVD Technique
Plasma enhanced chemical vapor deposition (PECVD) is a useful technique which has been employed to synthesize thin films from vapor state to solid state on the substrates. The plasma is generally originated by DC or RF power supply between showerhead electrode ordinarily used as top electrode and sample stage usually used as bottom electrode, respectively. The space between these two electrodes is filled with the chemical reactions from reactant gases. The crystal orientation and composition of the sample is dependent on the synthesis condition and the ratio of reactant precursors. For instance, the DEZn and CO2 as precursors were used to provide zinc and oxygen sources for synthesizing ZnO thin film, respectively. Obviously, the plasma is a main feature in this technique. Through the plasma enhanced assistance, ionized reactant atoms or molecules that effectively diffuse to the substrate surface, and then readily react with neighboring ionized atoms or molecules to form high dense configuration thin film. As a consequence, all the samples can be exposed to energetic ion bombardment during the synthesis process.
Because the plasma reaction is consecutively occurring during the synthesis process, the main benefits of PECVD technique include: (a) the lower synthesis temperature, almost all samples can be synthesized at low temperature (from 100 to 450 oC), (b) high aspect ratio condition is available (if use high density plasma), (c) high deposition rate, (d) RF powered showerhead with optimized gas delivery, provides uniform plasma processing, (e) good thin film surface uniformity, and (f) chemical composition structure can be finely controlled.
However, the PECVD technique involves chemical reaction and high density plasma bombardment, and it has some limitations and drawbacks. The whole process will produce a large number of chemical and particulate pollutions, required careful and appropriate handling. High pure gases are necessary in this technique, so the limitation of a specific precursor such as DEZn that can be processed in solution. If the source does not have the ability to dissolve in some solvent to prepare volatile precursor, the carrier gas will not deliver the reagent gas into the chamber to do synthesis. Moreover, although high density plasma bombardment can improve uniformity and quality of thin film, high residual stress will be created resulting in break of the thin film. Also, this technique needs an extra DC or RF power supply system leading to a little higher in cost compared with conventional chemical vapor deposition (CVD).
Future Directions and Applications
Many process parameters such as working pressure, gas composition, gas flow rate, RF power, reactant precursor and others are all interdependent for synthesizing thin films in the PECVD technique. Only the change of synthesis temperature has been studied in this presented research. There is still a lot of work requiring for further investigating the limitations and capabilities in this technique. For example, the gas composition could vary the stoichiometry of thin films and working pressure could affect the mean free path of ionized gases, which will yield diverse thin film configuration. Therefore, future work will require for further understanding how to manipulate the crystal quality or properties through above mentioned parameters.
The PECVD technique has been widely used in the manufacturing of the synthesis of thin films on the semiconductors which usually requires low process temperature. The PECVD method has been allowed and used to synthesize thin film on the polymer substrates, which also can be employed in flexible devices application. In addition to the low temperature application, we have already fabricated non-polar ZnO thin film by increasing the synthesis temperature, which could be used in high-efficiency Light-Emitting Diode (LED) or environmental sensor technology.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was financially supported of by the Ministry of Science and Technology and National Science Council of Republic of China (contract nos. NSC 101-2221-E-027-042 and NSC 101-2622-E-027-003-CC2). D. H. Wei thanks the National Taipei University of Technology (TAIPEI TECH) for the Dr. Shechtman Prize Award.
Materials
| RF power supply | ADVANCED ENERGY | RFX-600 | |
| Butterfly valve | MKS | 253B-1-40-1 | |
| Mass flow conctroller | PROTEC INSTRUMENTS | PC-540 | |
| Pressure conctroller | MKS | 600 series | |
| Heater | UPGRADE INSTRUMENT CO. | UI-TC 3001 | |
| Sputter gun | AJA INTERNATIONAL | A320-HA | |
| DEZn 1.5M | ACROS ORGANIC USA, New Jersey | also called Diethylzinc (C2H5)2Zn | |
| Spin coater | SWIENCO | PW – 490 | |
| I-V measurement | Keithley | Model: 2400 | |
| Photocondutive measurement | Home-built | ||
| UV light sourse | Panasonic | ANUJ 6160 | |
| Mask aligner | Karl Suss | MJB4 | |
| Photoresist | Shipley a Rohm & Haas company | S1813 | |
| Developer | Shipley a Rohm & Haas company | MF319 | |
| Silicon wafer | E-Light Technology Inc | 12/0801 | |
| Glass substrate | CORNING | 1737 | P-type / Boron |
References
- Choppali, U., Kougianos, E., Mohanty, S. P., Gorman, B. P. Influence of annealing on polymeric derived ZnO thin films on sapphire. Thin Solid Films. 545, 466-470 (2013).
- Bedia, F. Z., et al. Effect of tin doping on optical properties of nanostructured ZnO thin films grown by spray pyrolysis technique. J. Alloy. Compd. 616, 312-318 (2014).
- Liu, W. S., Wu, S. Y., Hung, C. Y., Tseng, C. H., Chang, Y. L. Improving the optoelectronic properties of gallium ZnO transparent conductive thin films through titanium doping. J. Alloy. Compd. 616, 268-274 (2014).
- Baik, K. H., Kim, H., Kim, J., Jung, S., Jang, S. Nonpolar light emitting diode with sharp near-ultraviolet emissions using hydrothermally grown ZnO on p-GaN. Appl. Phys. Lett. 103, 091107 (2013).
- Han, S. J., Huang, W., Shi, W., Yu, J. S. Performance improvement of organic field-effect transistor ammonia gas sensor using ZnO/PMMA hybrid as dielectric layer. Sens Actuator B-Chem. 203, 9-16 (2014).
- Chizhov, A. S., et al. Visible light activated room temperature gas sensors based on nanocrystalline ZnO sensitized with CdSe quantum dots. Sens Actuator B-Chem. 205, 305-312 (2014).
- Li, C., et al. Effects of substrate on the structural, electric and optical properties of Al-doped ZnO films prepared by radio frequency magnetron sputtering. Thin Solid Films. 517, 3265-3268 (2009).
- Ellmer, K. Resistivity of polycrystalline zinc oxide films: current status and physical limit. J. Phys. D: Appl. Phys. 34, 3097 (2001).
- Wang, F. G., et al. optical and electrical properties of Hf-doped ZnO transparent conducting films prepared by sol-gel method. J. Alloy. Compd. 623, 290-297 (2015).
- Senay, V., et al. ZnO thin film synthesis by reactive radio frequency magnetron sputtering. Appl. Surf. Sci. 318, 2-5 (2014).
- Chi, P. W., Su, C. W., Jhuo, B. H., Wei, D. H. Photoirradiation caused controllable wettability switching of sputtered highly aligned c-axis-oriented zinc oxide columnar films. Int. J. Photoenergy. 2014, 765209 (2014).
- Jamal, R. K., Hameed, M. A., Adem, K. A. Optical properties of nanostructured ZnO prepared by a pulsed laser deposition technique. Mater. Lett. 132, 31-33 (2014).
- Kobayashi, T., Nakada, T. Effects of post-deposition on transparent conductingZnO:B thin films grown by MOCVD. Jpn. J. Appl. Phys. 53, 05FA03 (2014).
- Chao, C. H., et al. Postannealing effect at various gas ambients on ohmic contacts of Pt/ZnO nanobilayers toward ultraviolet photodetectors. Int. J. Photoenergy. 2013, 372869-1155 (2013).
- Barankin, M. D., Gonzalez II, E., Ladwig, A. M., Hicks, R. F. Plasma-enhanced chemical vapor deposition of zinc oxide at atmospheric pressure and low temperature. 91, 924-930 (2007).
- Fons, P., et al. Uniaxial locked epitaxy of ZnO on the α face of sapphire. Appl. Phys. Lett. 77, 1801 (2000).
- Ko, H. J., Chen, Y., Hong, S. K., Yao, T. a. k. a. f. u. m. i. MBE growth of high-quality ZnO films on epi-GaN. J. Cryst. Growth. 209, 816-821 (2000).
- Park, D. J., Lee, J. Y., Park, T. E., Kim, Y. Y., Cho, H. K. Improved microstructural properties of a ZnO thin film using a buffer layer in-situ annealed in argon ambient. Thin Solid Films. 515, 6721-6725 (2000).
- Kim, M. S., et al. Nitrogen-passivation effects of Si substrates on the properties of ZnO epitaxial layers grown by using plasma-assisted molecular beam epitaxy. J. Korean Phys. Soc. 56, 827-831 (2010).
- Li, G. M., Zhang, J. W., Hou, X. Temperature dependence of performance of ZnO-based metal-semiconductor-metal ultraviolet photodetectors. Sens. Actuator A-Phys. 209, 149-153 (2014).
- Wang, X. F., et al. superhigh gain visible-blind UV detector and optical logic gates based on nonpolar a-axial GaN nanowire. Nanoscale. 6, 12009-12017 (2014).
- Inamdar, S. I., Rajpure, K. Y. High-performance metal-semiconductor-metal UV photodetector based on spray deposited ZnO thin films. J. Alloy. Compd. 595, 55-59 (2014).
- Tian, C. G., et al. Effects of continuous annealing on the performance of ZnO based metal-semiconductor-metal ultraviolet photodetectors. Mater. Sci. Eng. B-Adv. Funct.Solid-State Mater. 184, 67-71 (2014).
- Chen, H. Y., et al. Realization of a self-powered ZnO MSM UV photodetector with high responsivity using an asymmetric pair of Au electrodes. J. Mater. Chem. C. 2, 9689-9694 (2014).
- Subramanyam, T. K., Srinivasulu Naidu, ., S, S., Uthanna, Effect of substrate temperature on the physical properties of DC reactive magnetron sputtered ZnO films. Opt. Mater. 13, 239-247 (1999).
- Iwanaga, H., Kunishige, A., Takeuchi, S. Anisotropic thermal expansion in wurtzite-type crystals. J. Mater. Sci. 35, 2451-2454 (2000).
- Okaji, M. Absolute thermal expansion measurements of single-crystal silicon in the range 300-1300 K with an interferometric dilatometer. Int. J. Thermophys. 9, 1101-1109 (1988).
- Pearse, R. W. B., Lichtenberg, A. J. . The identification of molecular spectra. , (1976).
- Chao, C. H., Wei, D. H. Growth of non-polar ZnO thin films with different working pressures by plasma enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 53, 11RA05 (2014).
- Lin, B., Fu, Z., Green Jia, Y. luminescent center in undoped zinc oxide films deposited on silicon substrate. Appl. Phys. Lett. 79, 943-945 (2001).
- Koida, T., et al. Radiative and nonradiative excitonic transitions in nonpolar (110) and polar (000) and (0001) ZnO epilayers. Appl. Phys. Lett. 84 (110), 1079 (2004).

