A CO2 Concentration Gradient Facility for Testing CO2 Enrichment and Soil Effects on Grassland Ecosystem Function
Summary
The Lysimeter Carbon Dioxide Gradient Facility creates a 250 to 500 µl L-1 linear carbon dioxide gradient in temperature-controlled chambers housing grassland plant communities on clay, silty clay, and sandy soil monoliths. The facility is used to determine how past and future carbon dioxide levels affect grassland carbon cycling.
Abstract
Continuing increases in atmospheric carbon dioxide concentrations (CA) mandate techniques for examining impacts on terrestrial ecosystems. Most experiments examine only two or a few levels of CA concentration and a single soil type, but if CA can be varied as a gradient from subambient to superambient concentrations on multiple soils, we can discern whether past ecosystem responses may continue linearly in the future and whether responses may vary across the landscape. The Lysimeter Carbon Dioxide Gradient Facility applies a 250 to 500 µl L-1 CA gradient to Blackland prairie plant communities established on lysimeters containing clay, silty clay, and sandy soils. The gradient is created as photosynthesis by vegetation enclosed in in temperature-controlled chambers progressively depletes carbon dioxide from air flowing directionally through the chambers. Maintaining proper air flow rate, adequate photosynthetic capacity, and temperature control are critical to overcome the main limitations of the system, which are declining photosynthetic rates and increased water stress during summer. The facility is an economical alternative to other techniques of CA enrichment, successfully discerns the shape of ecosystem responses to subambient to superambient CA enrichment, and can be adapted to test for interactions of carbon dioxide with other greenhouse gases such as methane or ozone.
Introduction
Atmospheric carbon dioxide concentration (CA) has recently increased past 400 µl L-1 from approximately 270 µl L-1 prior to the Industrial Revolution. CA is forecast to reach at least 550 µl L-1 by 21001. This rate of increase surpasses any CA changes observed over the last 500,000 years. The unprecedented rate of change in CA raises the possibility of non-linear or threshold responses of ecosystems to increasing CA. Most ecosystem-scale CA enrichment experiments apply only two treatments, a single level of enriched CA and a control. These experiments have greatly expanded our understanding of the ecosystem impacts of CA enrichment. However, an alternate approach that can reveal the presence of non-linear ecosystem responses to increasing CA is to study ecosystems across a continuous range of subambient to superambient CA. Subambient CA is difficult to maintain in the field, and has most often been studied using growth chambers2. Superambient CA has been studied using growth chambers, open-top chambers, and free-air enrichment techniques3, 4.
CA enrichment occurs across landscapes containing many soil types. Soils properties can strongly affect ecosystem responses to CA enrichment. For example, soil texture determines the retention of water and nutrients in the soil profile5, their availability to plants6, and the amount and quality of organic matter7-9. The availability of soil moisture is a crucial mediator of ecosystem responses to CA enrichment in water limited systems, including most grasslands10. Past field CA enrichment experiments have typically examined only one soil type, and controlled tests of continuously varying CA enrichment over several soil types are lacking. If effects of CA enrichment on ecosystem processes differ with soil type, there is strong reason to expect spatial variation in ecosystem responses to CA enrichment and ensuing changes in climate11, 12.
The Lysimeter Carbon Dioxide Gradient (LYCOG) facility was designed to address questions of spatial variation in non-linear and threshold responses of ecosystems to CA levels ranging from ~ 250 to 500 µl L-1. LYCOG creates the prescribed gradient of CA on perennial grassland plant communities growing on soils representing the broad range of texture, N and C contents, and hydrologic properties of grasslands in the southern portion of the U.S. Central Plains. Specific soils series used in the facility are Houston Black clay (32 monoliths), a Vertisol (Udic Haplustert) typical of lowlands; Austin (32 monoliths), a high carbonate, silty clay Mollisol (Udorthentic Haplustol) typical of uplands; and Bastsil (16 monoliths), an alluvial sandy loam Alfisol (Udic Paleustalf).
The operational principle employed in LYCOG is to harness the photosynthetic capacity of plants to deplete CA from parcels of air moved directionally through the enclosed chambers. The treatment objective is to maintain a constant linear daytime gradient in CA from 500 to 250 µl L-1. To accomplish this, LYCOG consists of two linear chambers, a superambient chamber maintaining the portion of the gradient from 500 to 390 (ambient) µl L-1 CA, and a subambient chamber maintaining the 390 to 250 µl L-1 portion of the gradient. The two chambers are located side by side, oriented on a north-south axis. The CA gradient is maintained during the portion of the year when vegetation photosynthetic capacity is adequate; typically from late April to early November.
The chambers contain sensors and instrumentation needed to regulate the CA gradient, control air temperature (TA) near ambient values, and apply uniform precipitation amounts to all soils. Soils are intact monoliths collected from nearby Blackland prairie installed in hydrologically-isolated weighing lysimeters instrumented to determine all components of the water budget. Water is applied in events of volume and timing that approximate the seasonality of rain events and amounts during an average precipitation year. Thus, LYCOG is capable of evaluating the long-term effects of subambient to superambient CA and soil type on grassland ecosystem function including water and carbon budgets.
LYCOG is the third generation of CA gradient experiments conducted by USDA ARS Grassland Soil and Water Research Laboratory. The first generation was a prototype subambient to ambient gradient that established the viability of the gradient approach13 and advanced our understanding of leaf-level physiological responses of plants to subambient variation in CA14-20. The second generation was a field-scale application of the concept to perennial C4 grassland, with the gradient extended to 200 to 550 µL L-1 21. This field-scale experiment provided the first evidence that grassland productivity increases with CA enrichment may saturate near current ambient concentrations20, in part because nitrogen availability may limit plant productivity at superambient CA22. LYCOG extends this second generation experiment by incorporating replicated soils of varying texture, allowing robust testing for interactive effects of soils on the CA response of grassland communities.
Protocol
1. Collect Soil Monoliths to be used as Weighing Lysimeters
- Construct open-ended steel boxes 1 x 1 m square by 1.5 m deep from 8 mm thick steel.
- Press the open-ended boxes vertically into the soil, using hydraulic presses mounted on helical anchors drilled 3 m deep into the soil.
- Excavate the encased monolith using a backhoe or similar equipment.
- Place a fiberglass wick in contact with soil at the base of the monolith. Pass the wick through the steel base into a 10 L reservoir to drain the monolith, and then weld the steel base onto the bottom of the box.
- Kill existing vegetation on the monoliths by applying a non-residual herbicide, such as glyphosate.
2. Establish Plant Communities on Soil Monoliths
- Plant the monoliths with eight seedlings each of seven species of tallgrass prairie grasses and forbs, for a total density of 56 plants per m2.
- Plant the following Grasses: Bouteloua curtipendula (side-oats grama), Schizachyrium scoparium (little bluestem), Sorghastrum nutans (Indiangrass), Tridens albescens (white tridens)].
- Plant the following Forbs: Salvia azurea (pitcher sage), Solidago canadensis (Canada goldenrod), Desmanthus illinoensis (Illinois bundleflower, a legume).
- Plant seedlings in a Latin Square design, re-randomized for each monolith.
- Water the transplants for approximately 2 months following planting. The goal is to minimize water stress during initial establishment. Use any convenient method such as a hand wand or garden sprinkler. The frequency of watering depends on local climate and weather, particularly the occurrence of ambient rainfall.
- Following the initial transplant establishment phase, maintain the transplants under ambient rainfall for as long as necessary while chambers (Section 3) are constructed. Remove unwanted species that emerge in the monoliths during establishment by hand-weeding.
3. Chamber Design
- Construct two chambers each 1.2 m wide, 1.5 m tall, and 60 m long, divided into ten 5 m long sections. Construct sections from heavy steel of dimensions 5 m x 1.2 m x 1.6 m deep, buried to 1.5 m.
- Install four monoliths in each section, two monoliths each of two of the soil types, in random order. Install each monolith atop a 4,540 kg capacity balance.
- Include Bastsil monoliths in the pairings in even numbered sections.
- Join adjacent sections aboveground with a 1 m long x 1 m wide x 0.3 m tall sheet-metal duct to provide a pathway for airflow.
- Supply coolant at 10 °C from a 161.4 kW refrigeration unit to a cooling coil inside each duct.
- Enclose the vegetation with clear greenhouse film (thickness 0.006”/.15 mm), such as used in other climate manipulation experiments23.
- Fit each cover with a zippered opening backed by a draft flap to allow access to the monoliths for sampling.
- Remove the polyethylene covers at the end of the growing season.
4. CO 2 and Air Temperature Measurement; Temperature Control
- Sample entry and exit CA on both chambers every 2 min through filtered air sample lines located at the entry and exit of superambient and subambient chambers. These data inform CO2 injection and fan speed control.
- Sample CA and water vapor content, and measure air temperature (TA) at the entry and exit of each 5 m section at 20 min intervals.
- Measure all air samples for CO2 and water vapor content in real time using infrared gas analyzers according to manufacturer’s protocol.
- Measure TA at the entry, midpoint, and exit of each section with shielded fine wire thermocouples.
- Regulate the flow of coolant through the cooling coil at the entrance of each section to maintain a consistent mean (mid-section) TA from section to section near the ambient TA.
- Position a quantum sensor to have an unobstructed view of the sky and measure photosynthetic photon flux density according to manufacturer’s protocol. Light level is an input to the blower control algorithm.
5. C A Treatment Application
- Daytime
- Mix pure carbon dioxide (CO2) with incoming ambient air to 500 µl L-1 CA, using a mass flow controller in the entrance duct of the superambient leg. See Section 4 for CA measurement details.
- Advect the enriched air through the chambers using blower fans at the entrance to section 1 and in downstream sections.
- Maintain the desired exit CA of 390 µl L-1 (ambient air) by adjusting the blower speed.
- Increase the blower speed if the exit CA is below the set point. This allows less time for plant uptake of CO2, resulting in higher exit CA.
- Decrease the blower speed if exit CA is above the set point.
- Use the same approach in the subambient chamber except introduce ambient air and control to achieve exit CA of 250 µl L-1.
- Nighttime
- Reverse the direction of air flow.
- Inject CO2 into the daytime exit end of the superambient chamber to achieve 530 µl L-1 CA, and control advection rates to maintain 640 µl L-1 at the nighttime exit (daytime entrance.
- Introduce ambient air at ~ 390 µl L-1 CO2 into the nighttime entrance (daytime exit) of the subambient chamber and control advection rate to maintain 530 µl L-1 at the nighttime exit.
6. Precipitation Inputs
- Apply the mean growing season rainfall amount to each monolith.
- Supply water to each monolith from a domestic water source through a drip irrigation system. Schedule the irrigation events and application amounts to approximate the seasonal rainfall pattern for the experiment location. The exact schedule depends on local climate.
- Control application timing with a data logger and measure application volumes with flowmeters.
7. Sampling
- Measure vertical profiles of volumetric soil water content (vSWC) weekly during the period of CO2 control, with a neutron attenuation gauge or other appropriate probe.
- Recommended profile increments are 20 cm depth increments to 1 m depth, and one 50 cm increment below a 1 m.
- Measure monolith aboveground net primary productivity (ANPP) by harvesting all standing aboveground biomass at the end of the growing season.
- All aboveground biomass is removed each year, consequently standing biomass represents current primary production.
- Sort the sampled biomass by species, dry to constant mass, and weigh.
- Use biomass of individual species to quantify plant species contributions to ANPP.
Representative Results
The superambient and subambient portions of the gradient are maintained in separate chambers (Figure 1). However, over seven years of operation (2007 – 2013), the chambers maintained a linear gradient in CA concentration from 500 to 250 µl L-1 (Figure 2) with only a small discontinuity in CA between the exit of the enriched chambers (Monolith 40) and the entrance of the subambient portion of the gradient (Monolith 41).
Air temperature and vapor pressure deficit remained constant from section to section in both the superambient and subambient chambers, except in section 10 of the superambient chamber, and sections 19 and 20 of the subambient chamber, where air temperature averaged ~ 3 °C warmer than other sections (Figure 2). However there can be pronounced temperature increases of 5 – 7 °C within each section, and corresponding increases in vapor pressure deficit.
Averaged over the 2007 – 2013 growing seasons, vSWC varied linearly along the CA gradient on two of the three soils (Figure 3). vSWC in the top 20 cm of the soil profile increased by 3.1% per 100 µl L-1 increase in CA on the sandy loam (Bastsil series) soil (R2 = 0.34, p = 0.01), and by 1.7% per 100 µl L-1 CA on the clay soil (Houston series). However there was no change in 0-20 vSWC in the silty clay (Austin series) soil (p = 0.13).
Plant productivity also varied linearly with by CA, and the magnitude of the CA response differed among soils. ANPP (Figure 4A) of monoliths with Blackland prairie plant communities had the smallest response to CA on the clay soil, increasing by 59 g m-2 per 100 µl L-1 increase in CA (R2 = 0.22, p = 0.02). The ANPP response to CA enrichment was intermediate on the silty clay soil, increasing by 76 g m-2 per 100 µl L-1 of CO2 (R2 = 0.22, p = 0.02), and greatest on the sandy loam soil, where ANPP gained 131 g m-2 per 100 µl L-1 of CO2 (R2 = 0.55, p < 0.001).
These soil-specific responses of ANPP to CA corresponded closely to the soil-specific responses of a mesic C4 tallgrass, Sorghastrum nutans, the most abundant grass species in the experimental plant communities. Aboveground biomass of S. nutans increased most strongly with increased CA on the sandy loam soil, gaining over 200 g m-2 for every 100 µl L-1 increase in CA (R2 = 0.40, p = 0.005). In contrast, S. nutans gained only 100 g m-1 per 100 µl L-1 increase in CA on the silty clay soil (R2 = 0.50, p < 0.0001), while S. nutans responded marginally to CA on the clay soil (R2 = 0.12, p = 0.07; Figure 4B).
The soil-specific increase in ANPP with CA enrichment occurred despite decreases in productivity on the two more responsive soils by the xeric C4 mid-grass Bouteloua curtipendula (Figure 4C). B. curtipendula was the second most abundant species in the experimental communities. On the silty-clay soil, B. curtipendula was the dominant grass at subambient CA concentrations but decreased most strongly with CA enrichment on the silty clay soil (69 g m-2, per 100 µl L-1 increase in CA; R2 = 0.36, p < 0.008), decreased less strongly on the sandy loam soil (44 g m-2, per 100 µl L-1 increase in CA; R2 = 0.36, p = 0.008), and did not vary with CA enrichment on the clay soil (p = 0.46).
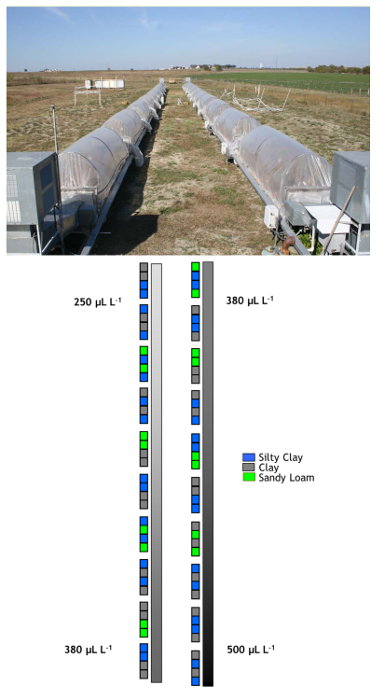
Figure 1. Arrangement of chambers and soils. The two linear sequences of chambers containing grassland vegetation growing on intact soil monoliths (photo), and schematic of the distribution of the three soil types along the CO2 gradient. Plot numbers 1 – 40 are located along the 500 – 380 µl L-1 portion of the gradient, and numbers 41 – 80 on the 380 – 250 µl L-1 portion. Photo: Philip Fay.
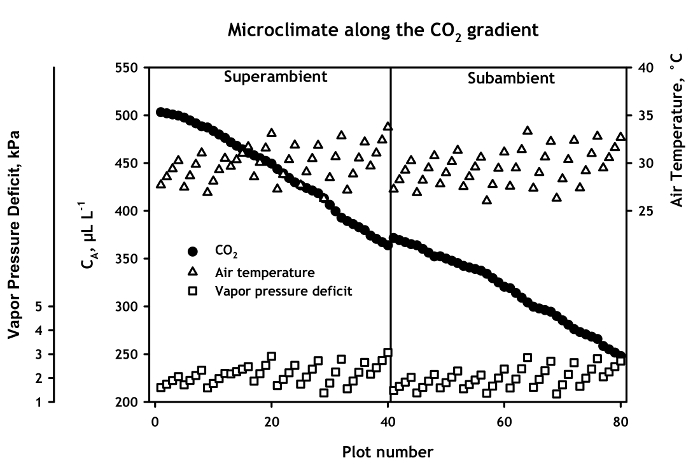
Figure 2. Microclimate along the CA gradient. Daytime growing season carbon dioxide (CO2) concentration, air temperature, and vapor pressure deficit for the 80 monoliths in the enriched and subambient chambers. Values are measured at the air entry and exit of each section, and estimated from linear interpolation for other positions. Data points represent means for the 2007 through 2013 growing seasons. Error bars omitted for clarity; mean standard errors were 3.5 for CO2, 0.82 for air temperature, and 0.18 for vapor pressure deficit.
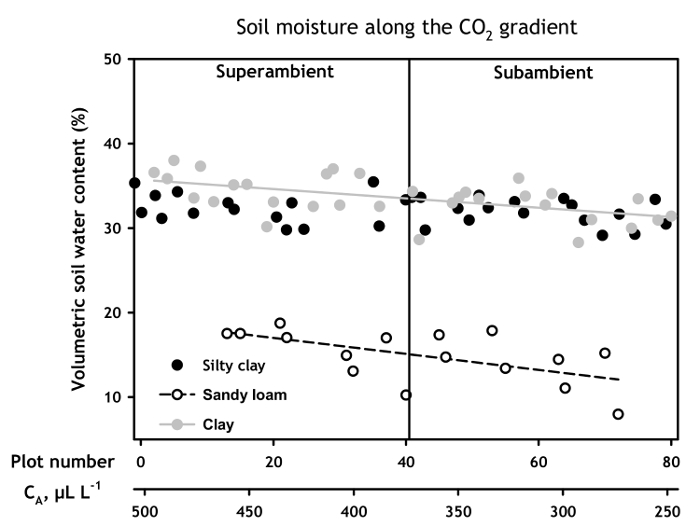
Figure 3. Soil moisture on each soil type along the CO2 gradient. Growing season volumetric soil water contents (vSWC) for 0 – 20 cm in the soil profile for each soil type, plotted by position along the CO2 concentration gradient. Linear regressions are plotted for soils with significant relationships of vSWC to CO2 concentration. Data points represent means of 2007 through 2013 growing seasons. Error bars omitted for clarity; mean standard errors on the three soils ranged from 0.74 to 0.99.
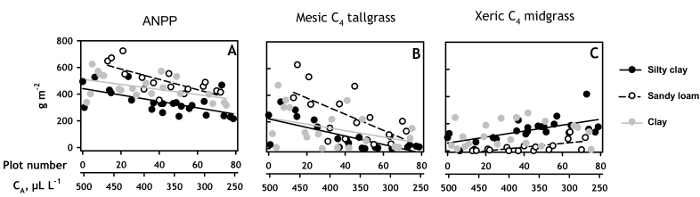
Figure 4. Plant productivity on each soil type along the CO2 gradient. (A) Mean aboveground net primary productivity (ANPP), the sum of the current-year biomass of all species in the 60 monoliths with Blackland Prairie plant communities; and the current year biomass of (B) the mesic C4 tallgrass, Sorghastrum nutans, and (C) the xeric C4 midgrass Bouteloua curtipendula plotted by position along the CO2 concentration gradient. Linear regressions are plotted for soils with significant relationships of ANPP or species biomass to CO2 concentration. Data points represent means of 2007 through 2013 growing seasons. Error bars omitted for clarity; mean standard errors on the three soils ranged from 34.9 to 42.5 for ANPP, 21.8 to 34.4 for S. nutans, and 7.4 – 24.8 for B. curtipendula.
Discussion
The LYCOG facility achieves its operational goal of maintaining a 250 to 500 µl L-1 continuous gradient of CA concentrations on experimental grassland communities established on three soil types. The change in CA is linear over the prescribed range. Air temperature increased within each section, but was reset by the between-section cooling coils in most sections. As a result, the operational goal of maintaining a consistent mean temperature from section to section was met over most of the gradient. Temperature and CA control are easily maintained during the spring and early summer when soil moisture is relatively high and plants are at their highest photosynthetic capacity.
Critical Steps in the Protocol
Control of blower speed is the most critical aspect of maintaining the prescribed CO2 gradient. Control is based on a combination of feedback and feed-forward techniques to match air flow to the vegetation carbon uptake. The feedback technique adjusts fan speed based on the difference between measured and target exit CO2 concentration. Feed-forward control anticipates changes in photosynthetic rate and rapidly (5 sec response time) adjusts fan speed, based on changes in photosynthetically active radiation measured with the quantum sensor. Feed-forward control considerably improves control over that achieved by feedback control alone. The maximum airflow rate through the chambers is on the order of 1 m sec-1, or about 3.6 km hr-1, which is on the low-end of wind speeds these plants see in the field. Thus, varying fan speed is unlikely to affect plant responses.
Another critical aspect of maintaining the CO2 gradient is the presence of adequate photosynthetic capacity. The steeper the gradient, the greater the canopy photosynthetic capacity required to draw down the CO2 concentration. Species or communities with more leaf area, higher photosynthetic rates, or longer chamber length all increase the CO2 draw-down that can be achieved. Care should also be taken the monolith volume and depth is chosen to provide a realistic rooting volume for the established plant communities. The species used here have rooting depths of 1 – 1.5 m, but other species may be shallower or deeper, and monolith volume should be adjusted accordingly. The final critical aspect is the importance of reliably supplying and controlling the flow of chilled water to the cooling coils between each section, in order to match chamber temperatures to outside diurnal and seasonal variation in outside ambient temperature.
Modifications to the Technique
The first year of operation revealed that the prairie vegetation was marginally capable of adequate CO2 drawdown. This was remedied by converting a total of 20 monoliths chosen from the Houston and Austin soil series to monocultures of switchgrass, Panicum virgatum. Switchgrass is a highly productive native tallgrass, and is well-watered throughout the growing season, which insures adequate C uptake capacity along the gradient even during the hot summer months. The first year also revealed greater than anticipated aerodynamic resistance in the chambers, which degraded flow rates in downstream chambers, leading to overheating. This issue was remedied by installation of additional downstream blower fans to boost the flow rates. We recommend installing new polyethylene covers each growing season to maintain maximum light transmittance.
Limitations of the Technique
The system poses certain operational issues that create both opportunities and limitations on the research questions the facility can support. Control of the gradient becomes more difficult from mid-summer through the end of the growing season, because higher summer temperatures lower soil moisture, increasing plant water stress and lowering photosynthetic capacity. This in turn requires slower air flow rates to achieve the CA draw-down needed to meet the target CA concentrations, which in turn further raises temperatures. This dynamic illustrates the limited ability of this system for studies of drought interactions with CO2 concentration. Temperature increases within each 5 meter section are unavoidable because of the linear flow design of the experiment. Long-wave energy accumulates within each chamber until air passes through the cooling coil and entering the next chamber. Within-section temperature increases are of similar magnitude to some of the higher estimates for future temperature increases expected with some climate change scenarios. Thus, the within-section temperature variation represents an opportunity to analyze grassland responses to interactions between CA and warming. Finally, the dimensions of the chamber limit the vegetation to a maximum height of about one meter, and the monolith area limits the vegetation to herbaceous species with smaller basal areas. The use of tree species, for example to study woody encroachment into grassland, would be impractical beyond the seedling stage.
Significance compared to Other Techniques
LYCOG is considerably more economical to operate compared to techniques such as FACE and OTC. LYCOG uses approximately 3,700 L per month of CO2, which is greater than the CO2 use in MiniFACE systems24, but much less than the CO2 consumption of FACE and OTC approaches3, 12. The major expense of maintaining the experiment comes from temperature control, which costs approximately $30,000 per year, comparable to estimates of the CO2 expense for open top chamber CA enrichment costs but still much less than that of the CO2 expense of Free Air CO2 Enrichment systems3. The economic advantages come in addition to the unique capability of supporting studies at subambient CO2 and along a continuous CO2 gradient.
Current and Future Applications
Current research is examining ecosystem responses other than ANPP, including soil CO2 efflux, and evapotranspiration, which will expand our understanding of soil-specific variation in CA effects on grassland carbon and water cycling. Future possibilities for research include combining temperature and CO2 treatments, for example by operating both chambers as superambient but maintaining one chamber at a warmer temperature differential with respect to ambient. Current vegetation can easily be replaced with other species or communities to study how variation in community structure influences CO2 effects on ecosystem function. Other ecologically important atmospheric constituents such as methane or ozone could be added to test for interactions with CO2.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Anne Gibson, Katherine Jones, Chris Kolodziejczyk, Alicia Naranjo, Kyle Tiner, and numerous students and temporary technicians for operating the LYCOG facility, conducting sampling, and data processing. L.G.R. acknowledges USDA-NIFA (2010-65615-20632).
Materials
| Dataloggers, multiplexers | Campell Scientific, Logan, UT, USA | CR-7, CR-10, CR-21X, SDM-A04, SDM-CD16AC, AM25T | |
| Thermocouples: Copper-constantan | Omega Engineering, Inc., Stamford, CT, USA | TT-T-40-SLE, TT-T-24-SLE | |
| Quantum sensor | Li-Cor Biosciences, Lincoln, NE, USA | LI-190SB | |
| CO2/H2O analyzer | Li-Cor Biosciences, Lincoln, NE, USA | LI-7000 | |
| Lysimeter scales | Avery Weigh-Tronix, Houston, TX, USA | DSL-3636-10 | |
| Air sampling pump | Grace Air Components, Houston, TX, USA | VP 0660 | |
| Dew-point generator | Li-Cor Biosciences, Lincoln, NE, USA | LI-610 | |
| Cold water chiller | AEC Application Engineering, Wood Dale, IL, USA | CCOA-50 | |
| Chilled water flow control values | Belimo Air Controls, Danbury, CT, USA | LRB24-SR | |
| Chilled-water cooling coils | Coil Company, Paoli, PA, USA | WC12-C14-329-SCA-R | |
| Carbon dioxide refrigerated liquid | Temple Welding Supply, Temple, TX, USA | UN2187 | |
| Polyethylene film | AT Plastics, Toronto, ON, Canada | Dura-film Super Dura 4 | |
| Blower motor/controller | Dayton Electric, Lake Forest, IL, USA | 2M168C/4Z829 | |
| Solenoids | Industrial Automation, Cornelius, NC, USA | U8256B046V-12/DC | |
| Leachate collection pump | Gast Manufacturing, Benton Harbor, MI, USA | 0523-V191Q-G588DX |
References
- . Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Climate Change 2013: The Physical Science Basis. , 1535 (2013).
- Gerhart, L. M., Ward, J. K. Plant responses to low CO2 of the past. New Phytol. 188 (3), 674-695 (2010).
- Kimball, B. A. Cost comparisons among free-air CO2 enrichment, open-top chamber, and sunlit controlled-environment chamber methods of CO2 exposure. Crit. Rev. Plant Sci. 11 (2-3), 265-270 (1992).
- Hendrey, G. R., Lewin, K. F., Nagy, J. Free Air Carbon Dioxide Enrichment: DevelopmentProgress, Results. Vegetatio. 104/105 (1), 16-31 (1993).
- Weng, E., Luo, Y. Soil hydrological properties regulate grassland ecosystem responses to multifactor global change: A modeling analysis. J. Geophys. Res. 113 (G3), G03003 (2008).
- Brady, N. C., Weil, R. R. . The Nature and Properties of Soils. , 960 (2002).
- Jenkinson, D. A. Studies on the decomposition of plant material in soil. V. The effects of plant cover and soil type opn the logg of carbon from 14C labelled ryegrass decomposing under field conditions. J. Soil Sci. 28 (3), 424-434 (1977).
- Hassink, J. Preservation of plant residues in soils differing in unsaturated protective capacity. Soil Sci. Soc. Am. J. 60 (2), 487-491 (1996).
- Oades, J. M. The retention of organic matter in soils. Biogeochemistry. 5 (1), 35-70 (1988).
- Knapp, A. K., et al. Consequences of more extreme precipitation regimes for terrestrial ecosystems. BioScience. 58 (9), 811-821 (2008).
- Ainsworth, E. A., Long, S. P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165 (2), 351-372 (2005).
- Rogers, A., Ainsworth, E. A., Kammann, C. F. A. C. E., Nosberger, J., Long, S. P., Norby, R. J., Stitt, M. Ch 24: Value: Perspectives on the Future of Free-Air CO2 Enrichment Studies. Managed Ecosystems and CO2: Case Studies, Processes, and Perspectives. Ecological Studies. 187, 431-449 (2006).
- Mayeux, H. S., Johnson, H. B., Polley, H. W., Dumesnil, M. J., Spanel, G. A. A controlled environment chamber for growing plants across a subambient CO2 gradient. Funct Ecol. 7 (1), 125-133 (1993).
- Polley, H. W., Johnson, H. B., Mayeux, H. S. Carbon dioxide and water fluxes of C3 annuals and C4 perennials at subambient CO2 concentrations. Funct Ecol. 6 (6), 693-703 (1992).
- Polley, H. W., Johnson, H. B., Mayeux, H. S., Malone, S. R. Physiology and growth of wheat across a subambient carbon dioxide gradient. Ann. Bot. 71 (4), 347-356 (1993).
- Polley, H. W., Johnson, H. B., Marino, B. D., Mayeux, H. S. Increase in C3 plant water-use efficiency and biomass over glacial to present CO2 concentrations. Nature. 361 (6407), 61-64 (1993).
- Polley, H. W., Johnson, H. B., Mayeux, H. S. Increasing CO2: comparative responses of the C4 grass Schizachyrium. and grassland invader Prosopis. Ecology. 75 (4), 976-988 (1994).
- Polley, H. W., Johnson, H. B., Mayeux, H. S. Nitrogen and water requirements of C3 plants grown at glacial to present carbon dioxide concentrations. Funct. Ecol. 9 (1), 86-96 (1995).
- Polley, H. W., Johnson, H. B., Mayeux, H. S., Brown, D. A., White, J. W. C. Leaf and plant water use efficiency of C4 species grown at glacial to elevated CO2 concentrations. Int. J. Plant Sci. 157 (2), 164-170 (2012).
- Polley, H. W., Johnson, H. B., Derner, J. D. Increasing CO2 from subambient to superambient concentrations alters species composition and increases above-ground biomass in a C3/C4 grassland. New Phytol. 160 (2), 319-327 (2003).
- Johnson, H. B., Polley, H. W., Whitis, R. P. Elongated chambers for field studies across atmospheric CO2 gradients. Funct. Ecol. 14 (3), 388-396 (2000).
- Gill, R. A., et al. Nonlinear grassland responses to past and future atmospheric CO2. Nature. 417 (6886), 279-282 (2002).
- Fay, P. A., Carlisle, J. D., Knapp, A. K., Blair, J. M., Collins, S. L. Productivity responses to altered rainfall patterns in a C4-dominated grassland. Oecologia. 137 (2), 245-251 (2003).
- Miglietta, F., et al. Spatial and temporal performance of the miniface (free air CO2 enrichment) system on bog ecosystems in northern and central Europe. Environmental Monitoring and Assessment. 66 (2), 107-127 (2001).

