Isolation of Giant Lampbrush Chromosomes from Living Oocytes of Frogs and Salamanders
Summary
We present simple techniques for isolating giant transcriptionally active lampbrush chromosomes from living oocytes of frogs and salamanders. We describe how to observe these chromosomes "alive" by phase contrast or differential interference contrast, and how to fix them for fluorescent in situ hybridization or immunofluorescent staining.
Abstract
We describe methods for studying the giant transcriptionally active lampbrush chromosomes (LBCs) found in the oocyte, or unlaid egg, of frogs and salamanders. Individual LBCs can be up to 1 mm in length and they reside in a gigantic nucleus, itself up to 0.5 mm in diameter. The large size of the chromosomes permits unparalleled observations of active genes by light optical microscopy, but at the same time special techniques are required for isolating the nucleus, removing the nuclear envelope, and spreading the chromosomes on a microscope slide. The oocyte nucleus, also called the germinal vesicle (GV), is isolated in a medium that allows partial gelling of the nuclear actin and preserves the delicate structure of the LBCs. This step is carried out manually under a dissecting microscope using jeweler's forceps. Next, the nuclear envelope is removed, again manually with jeweler's forceps. The nuclear contents are quickly transferred to a medium that disperses the actin gel and allows the undamaged LBCs to settle onto a microscope slide. At this point the LBCs and other nuclear organelles can be viewed by phase contrast or differential interference contrast microscopy, although finer details are obscured by Brownian motion. For high resolution microscopical observation or molecular analysis, the whole preparation is centrifuged to attach the delicate LBCs firmly to the slide. A brief fixation in paraformaldehyde is then followed by immunofluorescent staining or in situ hybridization. LBCs are in a transcriptionally active state and their enormous size permits molecular analysis at the individual gene level using confocal or super-resolution microscopy.
Introduction
Most vertebrates, with the notable exception of marsupials and placental mammals, produce large yolky eggs. Despite their sometimes enormous size, these eggs are single cells that reach their final dimension while still in the ovary of the female. Ovarian eggs are called oocytes and each typically contains a single giant nucleus, known since the early 19th century as the germinal vesicle or simply GV.1 Oocytes of the common laboratory frogs, Xenopus laevis and Xenopus tropicalis, reach a maximal diameter of 1.2 mm and 0.8 mm respectively (Figure 1). The GVs from mature oocytes of these two frogs are 0.3 – 0.4 mm in diameter (Figures 2, 3). Salamanders typically have even larger oocytes and GVs. Fully mature oocytes of the Mexican axolotl, Ambystoma mexicanum, are more than 2 mm in diameter and the GV is about 0.5 mm. Thus, these nuclei are readily visible to the naked eye and can be manipulated in many ways that are impossible with the nuclei of typical somatic cells.
Equally remarkable is the gigantic size of the chromosomes within the GV, a fact recognized already at the end of the 19th century. Individual chromosomes of Ambystoma and other salamanders can be up to 1 mm in length (Figures 4, 5). Those of Xenopus are considerable smaller, although with lengths up to 100 µm or more, they dwarf the typical somatic chromosomes of most organisms. An important feature of oocyte chromosomes is their extraordinary transcriptional activity, which leads to one of their most characteristic morphological features — hundreds of paired lateral loops (Figure 5). Each loop consists of one or a few transcription units that actively synthesize RNA. The loops give oocyte chromosomes a fuzzy appearance, which led to the name "lampbrush" chromosome after their superficial resemblance to the brushes used in earlier times to clean kerosene lamp chimneys.2
The focus of this paper is on the use of isolated GVs to study LBCs and nuclear organelles (nucleoli, histone locus bodies, and speckles). Two rather different techniques will be described. In the first, more common technique, GVs are isolated in a saline solution using jeweler's forceps, briefly rinsed to remove adherent yolk, and the nuclear envelope is removed, again with jeweler's forceps. The gelatinous contents, containing the LBCs and nuclear organelles, are allowed to settle onto a glass microscope slide or coverslip. Such preparations can be examined directly by phase contrast or DIC microscopy. Alternatively, preparations may be centrifuged to attach the LBCs and organelles to the slide or coverslip. Such preparations can then be processed for detailed molecular analysis of nucleic acids and proteins, primarily by immunofluorescence and fluorescent in situ hybridization (FISH).3-7
A second technique involves isolation of the GV in mineral oil.8 Oil-isolated GVs remain transcriptionally active for many hours and are potentially useful for studies where one wants the nuclear contents to be as lifelike as possible.9,10 Because the refractive index of the nuclear "sap" is close to that of the LBCs and other nuclear organelles (Figure 3), microscopical techniques can be a challenge with oil-isolated GVs.
Finally, because of their size and ease of manipulation, GVs are ideal material for studies on the nuclear envelope. The nuclear pore complex was first described from electron microscopic studies on amphibian GV envelopes11 and more recent superresolution observations have used the same material.12,13
Protocol
General information about frogs and salamanders, as well as sources of animals, can be found at the following websites: Xenbase (http://www.xenbase.org) and Sal-Site (http://www.ambystoma.org). This protocol follows the animal care guidelines of the Department of Embryology of the Carnegie Institution for Science.
1. Solutions
- Make 10 L "frog water": Add 10 mL of 1 M CaCl2 and 10 mL of 1 M NaHCO3 to 10 L deionized or dechlorinated H2O with stirring.
- Make 1 L 20% paraformaldehyde: Make 1 L of 4 mM Na2CO3. In a chemical hood add 200 g of powdered paraformaldehyde, and heat to about 80 oC to dissolve. Cool, and filter through filter paper. Caution: paraformaldehyde is toxic and must be handled with care. Alternatively, purchase commercial paraformaldehyde solution.
- Make 1 L amphibian anesthetic solution: Add 1.5 g of ethyl 3-aminobenzoate methanesulfonate to 1 L frog water. Store at room temperature.
- Make 1 L oocyte culture medium OR214: 82.5 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na2HPO4, 5 mM HEPES (from 500 mM stock, pH 8.3). The final pH will be about pH 7.8. Add 100 mg ampicillin and 100 mg streptomycin to prevent bacterial growth.
- Make 1 L GV isolation solution: 83 mM KCl, 17 mM NaCl, 6.5 mM Na2HPO4, 3.5 mM KH2PO4, 1 mM MgCl2, 1 mM dithiothreitol (DTT). Adjust to pH 7.0.
- Make 100 mL GV dispersal solution: 20.7 mM KCl, 4.3 mM NaCl, 1.6 mM Na2HPO4, 0.9 mM KH2PO4, 1 mM MgCl2, 1 mM dithiothreitol (DTT), 0.1% paraformaldehyde. Adjust to pH 7.0. To facilitate more rapid dispersal of the nuclear gel, add 10-50 µM CaCl2.
- Make 500 mL subbing solution. Swell 0.5 g of commercial gelatin in 20 mL H2O for 5 – 10 min. Add 500 mL boiling H2O and carefully swirl to dissolve the gelatin. Cool and add 50 mg CrK(SO4)2 ·12 H2O. Filter with suction and store at 4 oC.
- Make 10 mL mounting medium. Dissolve 10 mg phenylenediamine in 5 mL PBS (135 mM NaCl, 2.5 mM KCl, 4.3 mM Na2HPO4, 1.5 mM KH2PO4, adjusted to pH 9). Add 5 mL glycerol. Store in 250 µL aliquots at -80 oC.
2. Materials
- For subbed slides, place 3 inch x 1 inch glass microscope slides in a commercial detergent solution and rub them to remove any contaminants. Place in a slide rack and rinse well in tap water, then in deionized water. Dip the slides in the subbing solution, drain at an angle, and bake overnight at 60 oC.
- For plastic squares, cut 25 mm squares from a sheet of 1 mm thick poly(methyl methacrylate). Drill a 5 mm round hole in the center of each square. Sand both faces and the edges of the square as well as the circumference of the hole.
NOTE: As an alternative to homemade plastic squares and well slides, as described in the next paragraph, use commercial adhesive in situ PCR and hybridization chambers (25 µL). One could probably also use re-usable silicone isolators for cell culture, although this has not been thoroughly tested as yet. - For well slides, melt 20 – 30 g of paraffin wax (56 – 57 oC) in a 50 mL glass beaker on a hot plate. With a 20 µL pipette, spread one 5 µL drop of paraffin on each side of the center of a subbed slide. Press a plastic square into the paraffin and place the slide onto the hot plate. The paraffin will melt and should spread evenly under the plastic square.
- Remove the slide from the hot plate and let it cool. Place a support under each end of the slide, so that the slide does not contact the tabletop during the cooling process. If the paraffin cools too rapidly, it may pull away from the central hole. Prepare 10 or more well slides: each GV preparation uses a new well slide.
- For slide carriers for centrifugation, use specially constructed carriers for the rotor used. Several manufacturers provide centrifuges with carriers for 96 – well plastic plates. These carriers can be adapted quite easily to hold microscope slides.
3. Isolation of Oocytes
- Place an adult female frog (Xenopus laevis or Xenopus tropicalis) or salamander (Ambystoma mexicanum) in enough anesthetic solution to cover the animal completely. When the animal ceases movement, place it belly-up on a bed of chipped ice in a suitable container.
- With surgical scissors make a 2 – 3 cm incision in the lower abdomen lateral to the midline. Move the internal organs gently aside to find the ovary on the side of the incision (there are two ovaries, one on each side of the abdomen). Cut out a portion of the ovary with enough oocytes for the planned experiments. Since there are thousands of oocytes in each ovary, a relatively small piece of ovary (1 – 3 cm) will usually suffice.
- Place one or a few pieces of ovary in OR2 saline in a large petri dish (100 mm) at room temperature. Oocytes can be stored in good condition for several days, if damaged oocytes are removed and the OR2 solution is changed daily.
- Suture the incision with surgical silk and return the animal to an individual glass bowl for recovery. Add just enough "frog water" to cover the animal until it regains consciousness (about 15 min). After the animal shows voluntary movements, fill the bowl with water. Although fresh water should be used, sterility is not necessary, as amphibians almost never become infected after surgery. If desired, neomycin can be applied to the sutures. The wound usually heals completely after a few days and the same animal can be used again after about 2 months.
4. Isolation of a Germinal Vesicle (GV)
- Place a portion of ovary (10 – 20 large oocytes) in a small petri dish (35 x 10 mm) containing OR2 solution.
- Remove an oocyte from the ovary using two pairs of #5 jeweler's forceps to tear the thin stalk of tissue that connects the oocyte to the ovary wall. Place the oocyte in another small petri dish containing GV isolation medium.
NOTE: The state of the LBCs depends on the size (maturity) of the oocyte. LBCs reach maximal length with prominent loops in oocytes that are slightly less than full size. The most mature oocytes often contain short chromosomes with contracted loops. - Perform the following steps under low magnification of a dissecting microscope (field diameter approximately 10 – 20 mm).
- Grasp the oocyte with both pairs of forceps near the animal pole (dark hemisphere) and make a small tear. Look in the extruded yolk for the transparent oocyte nucleus, also called the germinal vesicle or GV.
- Alternatively, poke a large hole near the animal pole with one pair of forceps and gently squeeze out the GV along with yolk (Figure 2).
- Gently roll the GV away from the bulk of the yolk. Usually there will be a small amount of yolk tightly adherent to the GV.
5. Removal of the Nuclear Envelope
- For X. laevis and X. tropicalis, transfer the GV from the isolation medium to the spreading medium within 60 s of isolation, even if there is still a little adherent yolk.
NOTE: GVs of these two species "harden" within about 60 s if left in the isolation medium and will not spread in subsequent steps. Therefore speed is of the utmost importance when examining Xenopus GV contents.- Grasp the nuclear envelope near the top of the GV with one pair of jeweler's forceps, taking care not to press down. Grasp the envelope with a second pair of forceps as close to the first as possible. Pull the two pairs of forceps away from each other and the GV contents will "pop" out free of the envelope, which adheres tightly to one or both forceps.
- Pick up the GV contents in a glass pipette or an adjustable 20 µL micropipette. If using an adjustable pipette, set the pipette to 10 µL and cut off the end of the plastic tip with a razor blade. Draw in 5 µL of liquid before picking up the GV in the remaining 5 µL.
- Transfer the still-gelled nuclear contents to the center of a well slide previously filled with spreading solution. The spreading solution must have a convex surface so that a bubble is not trapped when the coverslip is added.
- Add a coverslip (18 mm) and place the slide in a moist chamber. The nuclear gel will slowly disperse over the next 10 – 60 min, so that the chromosomes and nuclear organelles come to lie on the surface of the glass slide.
NOTE: GVs of the salamanders A. mexicanum and N. viridescens do not "harden" unduly in the isolation medium. Therefore one can proceed more leisurely with removal of the nuclear envelope than one can with Xenopus.
- Remove the nuclear envelope from a salamander GV as described in section 5.1.1, but do so in the isolation medium.
- Pick up the slightly gelled GV contents with a pipette and transfer to a petri dish containing spreading solution.
- Quickly rinse out the pipette tip in the spreading solution, pick up the nuclear gel, and place it in the center of a well slide previously filled with spreading solution. Add an 18 mm coverslip and place the entire slide in a moist chamber. The nuclear sap will slowly disperse over the next 10 – 60 min and the chromosomes and nuclear organelles will come to lie on the glass surface.
6. Preliminary Observation of LBCs and Organelles
- View the LBCs and nuclear organelles by phase contrast (conventional upright microscope) at low magnification after dispersal of the nuclear contents in the spreading chamber. Because the chamber is approximately 1 mm deep and the nuclear contents are at the bottom, use a low magnification objective with a long working distance (5X – 10X).
NOTE: The major reason for examining the preparation at this time is to determine whether it is worth carrying through the next steps. In a good preparation the chromosomes are unbroken and have settled to the bottom of the chamber. Proceed to the centrifugation step within an hour of making the preparation, as this will ensure optimal attachment of the lampbrush chromosome loops to the slide.
7. Centrifugation of the Nuclear Contents
- Place a piece of filter paper on the coverslip and press down to remove excess liquid from the chamber.
- Put about 1 mm3 of petroleum jelly on each side of the coverslip. Melt the petroleum jelly with a metal rod heated to about 70 – 80 oC to seal the coverslip onto the underlying plastic square.
- Place slides in the slide carriers for centrifugation, making sure that opposite carriers are balanced. Centrifuge the slides at about 4,800 x g for 30 min – 1 h. at 4 oC. Use the slow start feature on the centrifuge.
8. Fixing the Nuclear Contents
- For most subsequent procedures, fix the nuclear contents with paraformaldehyde.
- Put a slide rack into a standard microscope slide staining dish and then place the slides individually in the rack. Add enough fixative to cover the slides(2 – 4% paraformaldehyde in OR2 or PBS).
- With a pair of blunt forceps, push the coverslip laterally, pick it up, and discard it in the appropriate glass trash. Leave slides in the fixative for at least 15 – 30 min. For in situ hybridization and most antibody stains, longer periods of fixation (h or d) are permissible.
- To remove the plastic square, place slides one at a time into ice cold OR2 or PBS in a staining dish. Insert a sharp razor blade at the edge of the plastic square and pry it loose.
NOTE: Ideally the plastic will come off cleanly, leaving all the paraffin wax on the slide. The wax serves a useful purpose: since it surrounds the small central area where the nuclear contents are attached to the slide, it acts as a dam that allows one to work with small volumes (5 – 10 µL) of reagents for immunostaining, etc. Slides can be held indefinitely in cold OR2 or PBS.
9. Immunostaining of LBCs and Nuclear Organelles
- Remove a fixed GV preparation from storage in OR2 or PBS. Wipe off excess liquid and place on supports in a moist chamber. A convenient chamber is a 90 x 15 mm petri dish containing a 90 mm circle of moist filter paper.
NOTE: At this point the nuclear contents are firmly attached to the slide in a 5 mm circular area free of paraffin wax. Because the wax is water-repellent, one can change solutions by adding and removing small volumes of liquid to the edge of the central area with a 20 µL pipette. The major precaution is to avoid touching the wax-free area with the pipette tip. - Carry out immunostaining with small volumes (10 – 20 µL) of reagents. Because the chromosomes and nuclear organelles are very small and in immediate contact with added reagents, staining protocols can be short. Primary and secondary antibody staining times can be as short as 1 hr or less. Do not include detergent in any step, as this causes damage to the preparation. If desired, stain the preparation with DAPI (1 µg/mL) for 10 – 20 min to accentuate the axes of the LBCs.
- Mount in glycerol or a commercial mounting medium suitable for immunofluorescence. To avoid trapping an air bubble during the mounting step, proceed as follows.
- Pull 15 µL of mounting medium into the tip of a 20 µL pipette. Remove as much liquid as possible from the area immediately around the nuclear spread by "wicking" it off with a piece of filter paper (cut in the shape of a very thin triangle from a 90 mm circle of #1 filter paper).
- Pipette most of the mounting medium onto the preparation, being careful not to touch the area of the nuclear spread. Place the last 1 µL or so of mounting medium in the center of a 22 mm coverslip (thickness #1.5). Invert the coverslip over the preparation and drop carefully into place.
- For temporary mounts, up to a few days, use 1 mg/mL phenylenediamine in 50% glycerol. For permanent mounts, especially for superresolution studies, use a low-fluorescence medium that hardens to a refractive index of about 1.46.
10. Fluorescent In Situ Hybridization (FISH) of LBCs and Nuclear Organelles
- Because FISH protocols usually require a step at elevated temperature, remove the paraffin wax from the preparation. Carefully scrape with a razor blade, although the procedure is tedious and can lead to accidental damage of the preparation. It is useful to mark the area of the preparation on the back of the slide with a diamond point.
- Alternatively, dissolve the paraffin wax with xylene. Set up a series of Coplin jars or staining dishes containing: 30%, 50%, 70%, 95%, and 100% ethanol; 3 containers of xylene; 2 more containers of 100% ethanol and one acetone.
- Place slides in the slide carrier and dehydrate them in the ethanol series, leaving slides 3 – 5 min in each concentration (shorter times if agitated).
- Dissolve the paraffin wax by passing the slides through the 3 containers of xylene, about 5 min each if agitated. Remove the xylene by passing through two 100% ethanols, and finally remove the ethanol with acetone. Take the slides from acetone and tilt them to dry.
- Carry out in situ hybridization using any standard protocol.15,16
11. Isolation of a GV under Oil
- Pick up an oocyte from the OR2 solution with jeweler's forceps, including as little surrounding tissue as possible. Place the oocyte on a small piece of #1 filter paper. Excess OR2 solution will transfer to the filter paper, leaving the oocyte almost free of liquid. Pick up the "dry" oocyte and place it under the surface of light weight mineral oil (paraffin oil) in a small petri dish (35 x 10 mm).
- With a fine tungsten needle or one tine of the forceps, poke a small hole in the oocyte near the dark animal pole. Pick up the oocyte with one pair of forceps (avoiding the tips) and squeeze gently. The GV will extrude along with a smaller or larger amount of yolk on its surface (Figure 3).
- Remove as much yolk as possible by gently sweeping the GV with the side of the forceps. In most cases it will be difficult to remove all of the yolk. However, for most purposes a small amount of yolk is not a problem.
- To observe the GV contents, flatten the GV to a greater or lesser extent.
- Remove the plastic square from a well slide and use the remaining paraffin wax as a shallow receptacle for paraffin oil.10 Pick up the GV along with paraffin oil in a 20 µL pipette and transfer to the center of the paraffin area. Add a coverslip and observe the contents of the partially flattened GV. This technique does not damage the LBCs.
- Alternatively, flatten the GV to approximately 10 µm thickness for observation of nuclear organelles.
- Cut off the final 1 mm of a plastic pipette tip with a sharp razor blade. Suck the GV into the tip along with exactly 5 µL of mineral oil. Extrude the GV and all the oil onto the center of a 22 mm glass coverslip.
- Lower a standard 3" x 1" glass slide slowly until it just touches the oil drop. The drop and coverslip will be lifted by capillarity and the oil will spread to the edges of the coverslip. When the drop has spread completely, the oil will be approximately 10 µm thick. The LBCs will be damaged and large nucleoli will be compressed slightly, but most of the smaller nuclear organelles will appear more or less as they exist in the intact GV (Figure 3).
Representative Results
To examine giant lampbrush chromosomes one begins by isolating oocytes from a frog or salamander. Figure 1 shows a group of mature oocytes in a buffered saline solution after removal from the ovary of the frog, Xenopus. Such oocytes remain in good condition for days at room temperature. The nucleus (or germinal vesicle) is then removed from an oocyte with jeweler's forceps, either in a saline solution (Figure 2) or in oil (Figure 3). The nuclear contents of an oil-isolated nucleus can be examined by gently squashing the nucleus and observing by phase contrast or differential interference contrast (DIC) microscopy. To examine the contents of a nucleus that has been isolated in a saline solution, one must first remove the nuclear envelope with jeweler's forceps and allow the contents to settle onto a microscope slide. The preparation is then centrifuged to attach the chromosomes firmly to the slide, after which the chromosomes can be stained with an antibody (Figure 4) or subjected to in situ hybridization. The lampbrush chromosomes of salamanders are much larger than those of frogs (Figure 5). In both cases individual transcription units (genes) can be seen as loops of chromatin projecting laterally from the chromosome axis.

Figure 1: Mature Oocytes from the Frog X. tropicalis. The ovary of a mature female frog contains thousands of oocytes in different stages of maturation. The smallest are the size of somatic cells and have already reached prophase of the first meiotic division. As the oocyte grows, it gradually accumulates yolk, which gives the cell an opaque white appearance. The largest mature oocytes, shown here, acquire a darker cap due to accumulation of melanin pigment. The largest oocytes of X. tropicalis are about 0.8 mm in diameter, those of X. laevis about 1.4 mm, while those of the axolotl are an enormous 2.2 mm. Except for size, all three are similar in general appearance. Scale bar = 1 mm.

Figure 2: Removal of the GV from an Oocyte of X. tropicalis. Left. A small hole was made with jeweler's forceps in the darker animal pole of an oocyte and the GV was gently extruded. In this example the GV was almost free of yolk as it came out of the oocyte. Adherent yolk can be removed by sucking the GV in and out of a pipette with a tip diameter just slightly larger than the diameter of the GV itself. Right. Three GVs of X. tropicalis. These GVs were left a few minutes in a slightly acid medium (GV isolation solution adjusted to pH 5.8), which causes the nuclear envelope to swell away from the gelled nuclear contents. GVs treated in this way will not spread for cytological examination. However, such GVs are ideal for molecular analysis: the envelope can be removed with jewelers forceps, providing a sample of nuclear contents completely free of cytoplasmic contamination.17,18 Scale bar = 0.5 mm. Please click here to view a larger version of this figure.
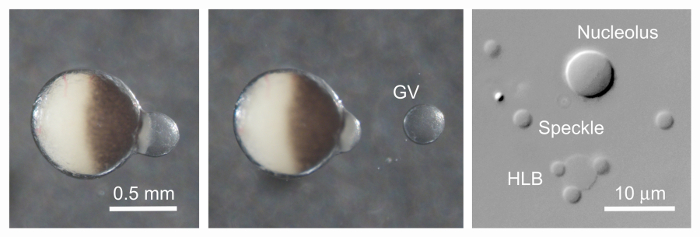
Figure 3: GV of X. tropicalis Isolated in Oil. Left. After a small puncture was made in the oocyte near the dark animal pole, the GV began to extrude. In this case the GV came out with almost no adherent yolk. Middle. The GV is now completely free from the oocyte cytoplasm. Such GVs continue to transcribe RNA for hours. Right. After the GV is gently squashed in oil under a coverslip, nuclear organelles can be viewed by DIC, as shown here, or by phase contrast. The entire GV is much larger than the small area shown here. HLB = histone locus body with three speckles on its surface. Scale bar = 0.5 mm for first two panels, 10 µm for the third. Please click here to view a larger version of this figure.
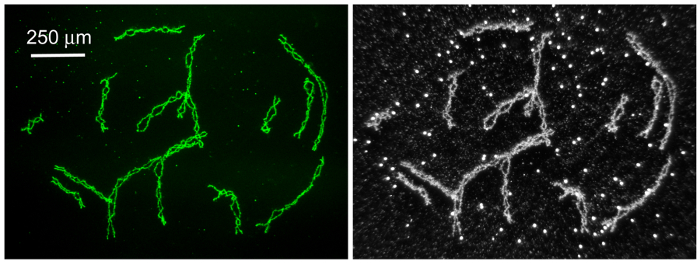
Figure 4: Lampbrush Chromosomes (LBCs) from the Axolotl A. mexicanum. Left. The 14 paired chromosomes from a single GV in prophase of the first meiotic division, immunostained with an antibody against phosphorylated RNA polymerase II. The tiny stained "dots" are histone locus bodies. Right. The same preparation viewed by darkfield illumination. One can now see the numerous unstained amplified nucleoli. Scale bar = 250 µm. Please click here to view a larger version of this figure.
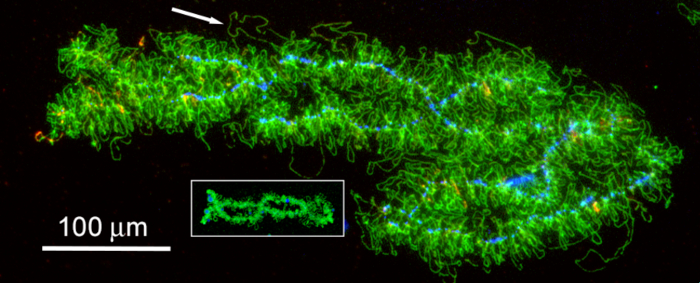
Figure 5: A Single LBC from the Axolotl A. mexicanum and the Frog X. tropicalis, at the Same Magnification. These images illustrate the extreme size difference between LBCs of a salamander and a frog (inset). The size difference correlates with the total DNA content of the genomes (about 30 Gbp for A. mexicanum vs 1.7 Gbp for X. tropicalis). Individual lateral loops (transcription units) are also much longer in the salamander than in the frog. Scale bar = 100 µm. Please click here to view a larger version of this figure.
Discussion
The first observations of "living" LBCs from hand-isolated GVs of frogs and salamanders were made nearly 80 years ago by the American biologist William Duryee,19 before the introduction of phase contrast and DIC microscopy, before fluorescent immunostaining, and before FISH. The advantages of LBCs for investigating details of chromosome structure and transcription at the individual gene level required development of techniques to attach the LBCs to glass slides, to fix them in a lifelike manner, and to apply molecular techniques without destroying their basic morphology. Nearly every technique to accomplish these goals requires some adaptation to the species under investigation and some compromise between lifelike preservation and molecular characterization. For instance, GVs and LBCs of tailed amphibians are unusually easy to work with because of their enormous size and the ease with which the GV contents disperse in the appropriate medium. Until recently, however, very little was known about their genomics, making it difficult to correlate the wealth of cytological features with molecular details. Conversely, LBCs of Xenopus are quite small compared to those of tailed amphibians (Figure 5), but the genomes of both X. tropicalis and X. laevis have been sequenced and are reasonably well annotated.
For those first using amphibian GVs, the major challenge will be to isolate the GV and remove the nuclear envelope without damaging the LBCs. To date, no one has discovered a way to remove the envelope except by hand with jeweler's forceps. It would be a major technical advance if a way were found to "dissolve" or "digest" the envelope but leave the chromosomes and other nuclear organelles undamaged. Such a technique would be equally useful for molecular studies. In our investigations on nuclear and cytoplasmic RNA we found it essential to remove the nuclear envelope before analysis of nuclear RNA (Figure 2).17,18 If the envelope is not removed, the small amount of nuclear RNA is overwhelmed by contaminating cytoplasmic RNA that adheres to the exterior of the envelope.
Another major improvement would be discovery of some way to attach LBCs more tightly to a microscope slide. Centrifugation of the preparation is critical, but even prolonged centrifugation (1 h at 4,500 x g) does not ensure attachment. One can usually tell when LBCs are well attached by examining them with phase contrast under medium magnification. Well-attached chromosomes show no Brownian motion at all. If any Brownian motion of the loops is detectable, subsequent immunostaining or FISH protocols will lead to extensive damage or loss of material.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01 GM33397. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.G.G. is American Cancer Society Professor of Developmental Genetics.
Materials
| Paraformaldehyde (reagent grade, crystalline) | Sigma-Aldrich | P6148-500G | Despite warnings in many protocols, a concentrated solution can be stored indefinitely at room temperature |
| Ethyl 3-aminobenzoate methanesulfonate | Sigma-Aldrich | A5040-100G | Sometimes referred to as MS-222 |
| Ethicon RB-1 1/2 circle taper point 3-0 sutures | VWR | 95057-000 | |
| Paraplast (paraffin wax) | Sigma-Aldrich | P3558-1KG | |
| p-Phenylenediamine | Sigma-Aldrich | P6001 | |
| Gelatin | Grocery Store | Commercial Knox gelatin works fine | |
| ProLong Gold antifade mountant | ThermoFisher Scientific | P10144 | |
| Gold Seal cover glass 22 x 22 mm #1 1/2 (0.16-0.19 mm thick) | Electron Microscopy Sciences | 63786-01 | These coverslips are the recommended thickness for superresolution microscopy |
| Dumont forceps #5 | Electron Microscopy Sciences | 72700-D | http://www.emsdiasum.com/microscopy/products/tweezers/dumont_positive_action.aspx |
| Paraffin oil (light) | EMD Chemicals | PX0047-1 | For isolating GVs in oil |
| Adhesive in situ PCR and hybridization chambers (25 µl). | BioRad | Frame-Seal Slide Chambers #SLF0201 | http://www.bio-rad.com/en-us/sku/slf0201-frame-seal-slide-chambers |
| Silicone isolators | Grace-Biolabs | select from catalog link | http://www.gracebio.com/life-science-products/microfluidics/silicone-isolators.html |
| Coplin jars and staining dishes | Electron Microscopy Sciences | select from catalog link | http://www.emsdiasum.com/microscopy/products/histology/staining.aspx |
References
- Purkinje, J. E. . Symbolae ad ovi avium historiam ante incubationem. , (1830).
- Rückert, J. E. Zur Entwickelungsgeschichte des Ovarialeies bei Selachiern. Anat. Anz. 7, 107-158 (1892).
- Callan, H. G. . Lampbrush Chromosomes. 36, (1986).
- Gall, J. G., Callan, H. G., Wu, Z., Murphy, C., Kay, B. K., Peng, H. B. Lampbrush chromosomes. Xenopus laevis: Practical Uses in Cell and Molecular Biology. Vol. 36 Methods in Cell Biology , 149-166 (1991).
- Gall, J. G., Wu, Z. Examining the contents of isolated Xenopus germinal vesicles. Methods. 51, 45-51 (2010).
- Penrad-Mobayed, M., Kanhoush, R., Perrin, C. Tips and tricks for preparing lampbrush chromosome spreads from Xenopus tropicalis oocytes. Methods. 51, 37-44 (2010).
- Morgan, G. T. Working with oocyte nuclei: cytological preparations of active chromatin and nuclear bodies from amphibian germinal vesicles. Methods Mol. Biol. 463, 55-66 (2008).
- Paine, P. L., Johnson, M. E., Lau, Y. T., Tluczek, L. J., Miller, D. S. The oocyte nucleus isolated in oil retains in vivo structure and functions. Biotechniques. 13, 238-246 (1992).
- Handwerger, K. E., Cordero, J. A., Gall, J. G. Cajal bodies, nucleoli, and speckles in the Xenopus oocyte nucleus have a low-density, sponge-like structure. Mol Biol Cell. 16, 202-211 (2005).
- Patel, S., Novikova, N., Beenders, B., Austin, C., Bellini, M. Live images of RNA polymerase II transcription units. Chromosome Res. 16, 223-232 (2008).
- Gall, J. G. Octagonal nuclear pores. J Cell Biol. 32, 391-399 (1967).
- Löschberger, A., et al. Super-resolution imaging visualizes the eightfold symmetry of gp210 proteins around the nuclear pore complex and resolves the central channel with nanometer resolution. J Cell Sci. 125, 570-575 (2012).
- Gottfert, F., et al. Coaligned dual-channel STED nanoscopy and molecular diffusion analysis at 20 nm resolution. Biophys J. 105, L01-L03 (2013).
- Wallace, R. A., Jared, D. W., Dumont, J. N., Sega, M. W. Protein incorporation by isolated amphibian oocytes: III. Optimum incubation conditions. J. Exp. Zool. 184, 321-333 (1973).
- Bridger, J., Spector, D. L., Goldman, R. D., Leinwand, L. A. Fluorescence in situhybridization to DNA. Cells: A Laboratory Manual. 3, 111.111-111.136 (1998).
- Singer, R. H., Spector, D. L., Goldman, R. D., Leinwand, L. A. In situ hybridization to RNA. Cells: A Laboratory Manual. 3, 111-116 (1998).
- Gardner, E. J., Nizami, Z. F., Talbot, C. C., Gall, J. G. Stable intronic sequence RNA (sisRNA), a new class of noncoding RNA from the oocyte nucleus of Xenopus tropicalis. Genes Dev. 26, 2550-2559 (2012).
- Talhouarne, G. J., Gall, J. G. Lariat intronic RNAs in the cytoplasm of Xenopus tropicalis oocytes. RNA. 20, 1476-1487 (2014).
- Duryee, W. R. Isolation of nuclei and non-mitotic chromosome pairs from frog eggs. Arch. Exp. Zellforsch. 19, 171-176 (1937).

