Nanostructured Ag-zeolite Composites as Luminescence-based Humidity Sensors
Summary
A protocol for the synthesis of moisture-responsive luminescent Ag-zeolite composites is described in this report.
Abstract
Small silver clusters confined inside zeolite matrices have recently emerged as a novel type of highly luminescent materials. Their emission has high external quantum efficiencies (EQE) and spans the whole visible spectrum. It has been recently reported that the UV excited luminescence of partially Li-exchanged sodium Linde type A zeolites [LTA(Na)] containing luminescent silver clusters can be controlled by adjusting the water content of the zeolite. These samples showed a dynamic change in their emission color from blue to green and yellow upon an increase of the hydration level of the zeolite, showing the great potential that these materials can have as luminescence-based humidity sensors at the macro and micro scale. Here, we describe the detailed procedure to fabricate a humidity sensor prototype using silver-exchanged zeolite composites. The sensor is produced by suspending the luminescent Ag-zeolites in an aqueous solution of polyethylenimine (PEI) to subsequently deposit a film of the material onto a quartz plate. The coated plate is subjected to several hydration/dehydration cycles to show the functionality of the sensing film.
Introduction
Small sub-nanometer oligoatomic silver clusters formed by self-assembly in confined zeolite matrices display unique optical properties.1-5 Such silver-zeolite composites have high chemical and photo-stability. However, their photoluminescence properties are highly dependent on the local environment of the silver clusters. The environmental conditions that influence the optical features in silver-zeolite composites can be divided into intrinsic and extrinsic properties. Intrinsic properties are related to the zeolite topology, the type of counter-balancing ions, and the silver loading.1 On the other hand, extrinsic properties are associated to the post-synthetic changes, such as the presence of adsorbates or water molecules in the zeolite cavities.3,4 The latter properties confer to silver-zeolite composites the ability to optically respond to external stimuli, such as variations of moisture within the zeolite scaffold6-8 or the presence of determined gases; therefore their use as water vapor and gas sensors has been suggested.9,10
In a recent study we have demonstrated that the optical response of Ag-zeolites to moisture is not only correlated to changes in the absorption or quenching of their emission but also to the appearance of different emission colors with respect to their water content.5 The stabilization of silver clusters in partially Li exchanged LTA zeolites led to the formation of a moisture-responsive material in which changes in the relative low humidity scale were reflected in a dynamic color change from a blue to green/yellow emission in dehydrated and hydrated samples, respectively. Therefore the use of these materials as luminescence-based humidity sensors was proposed. To date, different types of materials such as electrolytes, ceramics, polymers, and nanostructured composites have been proposed for monitoring changes in humidity based on electrical and optical responses.11,12 In this detailed protocol we aim to demonstrate a proof-of-concept for the application of LTA(Li)-Ag zeolites as humidity sensors and for further prototype developments. Due to the versatility of LTA(Li)-Ag zeolites to be incorporated into different substrates, their potential scalability and cost-effective fabrication, the prototype design might be facilitated.13 Such sensors could have potential applicability in different industrial sectors, such as in agriculture, as well as the automobile and paper industry.14
Protocol
Caution: The chemicals and reagents used in this report were handled with care using the appropriate safety protections (lab coats, gloves, safety goggles, fume hoods). This study deals with the manipulation of microporous inorganic materials (zeolites with sizes ranging from 1 to 5 microns), therefore special attention was directed to the use of adequate dust protection (dust masks). We recommend the consultation of the relevant material safety data (MSDS) of the chemicals and reagents employed in this work before use for proper manipulation and/or waste disposal.
1. Zeolite Pre-treatment
- Heat pre-treatment
NOTE: Pre-treat the zeolite materials before use to remove impurities, such as organic impurities, that could hinder the silver cluster formation and luminescence.- Weigh 10 g of commercial LTA(Na) zeolites (commercial LTA zeolites contain sodium as counter-balancing ions in their frameworks) and deposit it homogeneously on a porcelain tray.
- Heat the zeolite powder overnight in a muffle oven at 450 °C using a temperature ramp of 5 °C/min with intervals of 1 hr at 80 °C and 110 °C to avoid zeolite structure damage.
- Remove the zeolite material from the oven and let it cool down to room temperature under ambient conditions.
- Size selection of zeolite particles
NOTE: This will generate a more uniform grain size distribution of the starting zeolite materials, necessary for the creation of a homogenous film. This step also removes large amorphous particles, which are often present in industrially produced zeolites.- Weigh 10 g of commercial LTA(Na) and suspend it into 1 L of deionized water.
- Sonicate the suspension for 1 hr, vigorously shaking the suspension by hand every 10 min.
- Pour the suspension into an Atterberg cylinder (1 L) for 30 min. Particles smaller than 10 µm in size remain in suspension, but larger particles precipitate.
- Decant the suspension and recover the powder by filtration using a Büchner funnel. Wash the recovered powder three times with deionized water.
- Heat treat the powder as described in step 1.1.2.
2. Preparation of Luminescent LTA(Na)-Ag Zeolite Composites
- Synthesis of luminescent silver exchanged LTA zeolite [LTA(Na)-Ag] as reference material
- Dissolve 74.8 mg of silver nitrate in 200 ml deionized water in a 250 ml high density polypropylene (HDPE) bottle.
- Weigh 1 g of the pre-treated LTA(Na) sample and suspend it in the silver nitrate solution.
- Leave the HDPE flask agitating overnight into an end-over-end shaker oven at room temperature.
- Filter the suspension using a Büchner funnel and wash the zeolite powder 3 times with deionized water.
- Heat the recovered powder in a muffle oven at 450 °C, using the same procedure as described in step 1.1.2.
- Cool down the sample and place it in a desiccator with controlled humidity (98% relative humidity). Control the relative humidity by placing a saturated potassium sulfate solution inside the desiccator.15
- Measure the excitation and emission spectra of the samples (at different wavelengths) using a spectrofluorometer as well as their external quantum efficiencies.
- Measure two dimensional excitation-emission plots by placing the sample in a 1 mm path quartz cuvette. Collect emission spectra starting 30 nm above the excitation wavelength up to 800 nm using 5 nm steps and a dwell time of 0.1 sec.
- Apply corrections using the instrument software for the lamp intensity and the wavelength dependent detection of the emission path to the raw data. Additionally, use a long pass filter to avoid second order peaks in the two dimensional plots.
- Perform quantum efficiency measurements by using an integrating sphere attached to the spectrofluorometer.16 Record the emission scan from 240 nm to 600 nm for both the zeolite sample and BaSO4 reference using 260 nm as excitation wavelength, and then calculate the quantum efficiency using the instrument software.
3. Preparation of Luminescent [LTA(Li)-Ag] Zeolite Composites
- Synthesis of partially exchanged lithium LTA zeolite [LTA(Li)]
Note: The procedure followed for the fabrication of partially exchanged LTA(Li) zeolites was adapted from the report by Yahiro and collaborators.17- Dissolve 17.2 g lithium nitrate in 2.5 L deionized water.
- Pour 0.5 L of the lithium nitrate solution into a 1 L HDPE flask.
- Weigh 3 g of pre-treated LTA(Na) zeolite and suspend it in the HDPE flask containing the lithium nitrate solution.
- Agitate the flask using an end-over-end shaker oven at room temperature overnight.
- Filter the suspension using a Büchner funnel and wash the recovered powder 3 times with deionized water.
- Perform lithium exchange
- Add 0.5 L of fresh lithium nitrate solution (3.1.1) to a 1 L HDPE flask containing the recovered powder from the filtration step (3.1.5).
- Repeat steps 3.1.4 and 3.1.5.
- Repeat steps 3.1.6.1 and 3.1.6.2 another 4 times.
- Recover the zeolite powder and heat it in a muffle oven at 450 °C overnight using a temperature ramp of 5 °C/min with intervals of 1 hr at 80 °C and 110 °C.
- Synthesis of luminescent [LTA(Li)-Ag] zeolites
- Dissolve 74.8 mg silver nitrate in 200 ml deionized water using a 250 ml HDPE bottle.
- Weigh 1 g of the partially exchanged lithium LTA zeolite [LTA(Li)] and suspend it in the silver nitrate solution (3.2.1).
- Agitate the HDPE flask using an end-over-end shaker oven at room temperature overnight.
- Filter the suspension using a Büchner funnel and wash the recovered zeolite powder 3 times with deionized water.
- Heat-treat the powder in a muffle oven at 450 °C overnight using a temperature ramp of 5 °C/min with intervals of 1 hr at 80 °C and 110 °C.
- Cool down the sample under controlled humidity conditions using a desiccator containing a saturated potassium sulfate solution inside (98% relative humidity).15
- Measure the excitation and emission spectra of the samples as well as their external quantum efficiencies following the procedure described in step 2.1.7.
- Perform thermogravimetric analysis (TGA) to determine the water content in the sample at different temperatures.1 Briefly, place 30 to 50 mg of the as-prepared sample on a platinum sample holder and load it into the TGA device. Measure the weight loss from 50 °C until 600 °C using a heat rate of 5 °C/min under a nitrogen flow (90 ml/min).
4. Fabrication of a LTA(Li)-Ag / Polyethylenimine (PEI) Composite Deposited Film for Humidity Sensing Applications
Note: The deposition procedure used in this report was modified and adapted from reference 18.
- LTA(Li)-Ag colloidal suspension preparation.
- Dilute 1 ml of the commercial 50 wt% PEI solution to 100 ml with deionized water.
- Weigh 250 mg of the luminescent LTA(Li)-Ag material.
- Mix the zeolite and PEI solution together in a 125 ml HDPE bottle and shake the suspension vigorously.
- Place the bottle in a 40 kHz sonicator bath at room temperature overnight, to obtain a homogeneous suspension.
- Pour the LTA(Li)-Ag / PEI suspension into a spray bottle.
- Deposition of a LTA(Li)-Ag / PEI film onto a quartz plate for sensor prototype production.
- Clean a quartz plate by rinsing it with deionized water and acetone consecutively, prior to the film deposition. Dry the clean plates in an oven at 80 °C for 1 hr.
- Spray coat the quartz plate on one side, by placing the quartz plate horizontally on a clean sheet of aluminum foil and spraying three times (3 sec each time) from a distance of about 20 cm. Place the coated plate inside a drying oven at 50 °C for 30 min.
- Repeat step 4.2.2 another 4 times until the film is uniform.
- Hydration/dehydration of the sensor prototype.
- Place the coated quartz plate into the sample compartment of an in-house heating/vacuum cell.5
- Close the sample chamber of the cell by placing a clean quartz plate in combination with a rubber ring on top of the coated plate and seal the cell using a Teflon stopper and screws as depicted in Figure 2.
- Apply high vacuum, using a pressure below 10-3 mbar, to the cell overnight in order to dehydrate the sample.
- Visually monitor the emission color changes (in the visible region) of the deposited film by using a UV lamp.
- Open the sample chamber to monitor the emission color changes, in the visible region, upon rehydration of the film using a UV lamp.
- Repeat the cycle multiple times starting from step 4.3.2 to 4.3.5 to test the reversibility of the LTA(Li)-Ag/PEI film.
Representative Results
SEM micrographs of the LTA-Ag zeolite were recorded after the cation exchange and heat-treatment step. Subsequently the photoluminescence two-dimensional (2D) excitation/emission plots were measured for both the hydrated LTA(Na)-Ag and LTA(Li)-Ag zeolites (Figure 1). Elemental analysis was performed by XPS on the Ag exchanged zeolites to determine their chemical composition. The analysis shows that silver exchange on LTA(Na) and LTA(Li) zeolites is very close with a silver weight percentage of 19.6 wt% and 21.5 wt%, respectively. The difference in weight percentage could be ascribed to the lower atomic weight of Li atoms. Furthermore the elemental analysis also showed that after Li exchange 33% of Na is replaced. The cation exchange and subsequent heat-treatment step performed on the samples do not seem to affect the structure of the LTA crystals, as demonstrated by SEM. Moreover, the formation of larger silver nanoparticles on the surface of the zeolite crystals was not visualized. The luminescent properties largely differ between both the LTA(Li)-Ag and LTA(Na)-Ag samples in their hydrated state. By incorporating lithium into the zeolite framework as a counter-balancing cation, a blue shift in the excitation maximum occurs from 370 nm to 260 nm, for LTA(Na)-Ag and LTA(Li)-Ag, respectively. In contrast the emission maximum undergoes a small red shift from 550 to 565 nm by adding Li into the system. The largest difference between these samples is observed in their external quantum efficiencies (EQE). LTA(Na)-Ag zeolites possess an EQE of about 4% at its excitation maximum (370 nm), whereas the EQE for LTA(Li)-Ag zeolites reaches 62% (when excited at 260 nm). This results in a bright yellow emitting powder under 254 nm UV- illumination.
The luminescent properties of the LTA(Li)-Ag sample are also dependent on the water content of the system. This was shown by a combination of TGA and temperature dependent luminescence experiments, TGA correlates temperature to hydration level of the zeolite. Additionally, the temperature was indirectly related to the emission color displayed by the LTA(Li)-Ag sample by using an in-house heating cell (Figure 2). The emission color shifts from yellow over green to blue, when removing water from the LTA(Li)-Ag system. The EQE steadily drops from 62% (hydrated state) to 21% (dehydrated state).
Because of the water-responsive behavior of LTA(Li)-Ag, this material was used to fabricate a luminescence based humidity sensor prototype by suspending the powder into a PEI solution and subsequently spray coating the composite onto a quartz plate. Pictures (under daylight and UV illumination) and SEM micrographs of the spray-coated LTA(Li)-Ag/PEI film are displayed in Figure 3. We observed that by using this coating procedure, a relatively homogenous layer of the polymer-zeolite composite in terms of luminescence was obtained. The SEM micrograph shows that the zeolite crystals are not altered by the coating procedure. By using an in-house heating/vacuum cell it was also demonstrated that the polymer-zeolite film retains the water-responsive properties which were observed in the zeolite in powder form.
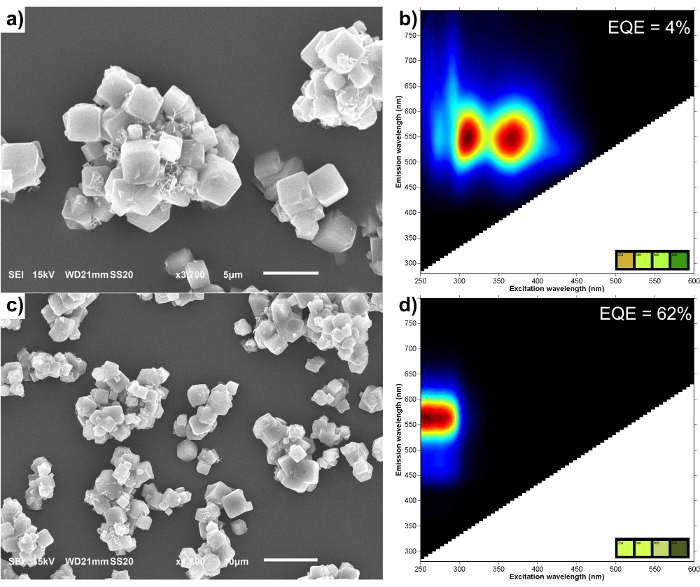
Figure 1: SEM pictures and luminescent properties of silver exchanged LTA zeolites. SEM micrographs and 2D excitation-emission plots of LTA(Na)-Ag (a,b) and LTA(Li)-Ag (c,d). The insets in the 2D excitation-emission plots display the simulated emission colors of the samples under different excitation wavelengths (254, 300, 366 and 450 nm). Please click here to view a larger version of this figure.
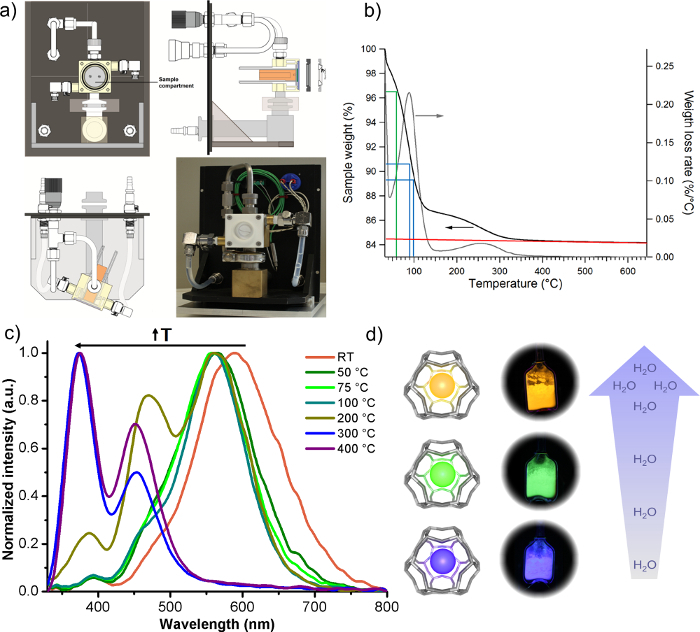
Figure 2: Effect of hydration level on the luminescent properties of LTA(Li)-Ag. a) Schematic representation of the in-house heating/vacuum cell employed in this study. b) TGA plot for the LTA(Li)-Ag sample. c) Normalized emission spectra (upon 260 nm excitation) of the LTA(Li)-Ag sample measured at different temperatures. d) Scheme displaying the emission color change of real samples with respect to the water content. Please click here to view a larger version of this figure.
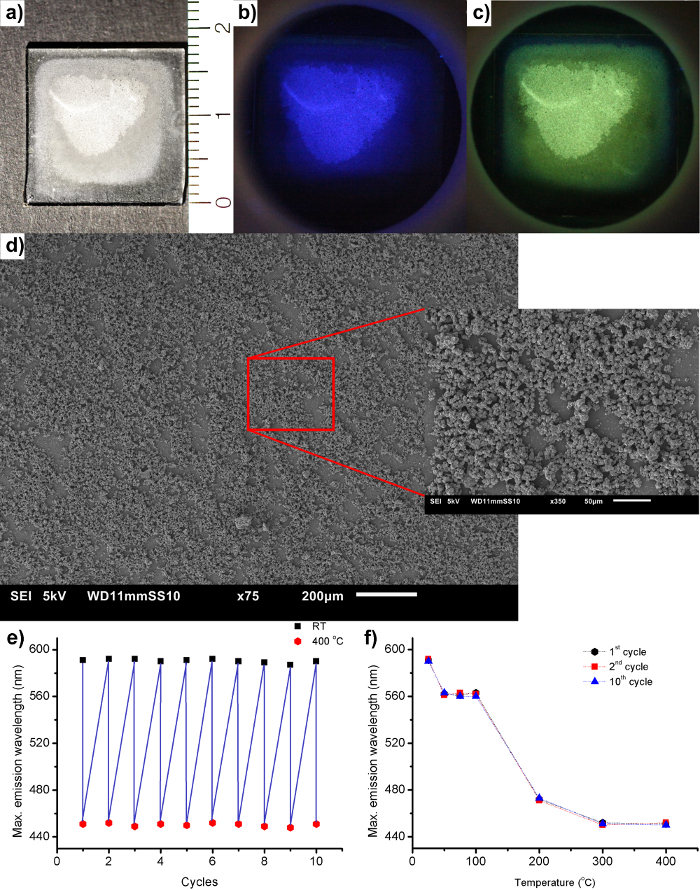
Figure 3: Luminescent humidity sensor based on a LTA(Li)-Ag/PEI composite. a) Photographs of the coated plate under daylight illumination. (b,c) Pictures of the dehydrated and hydrated coated plate under 254 nm UV-light irradiation, respectively. d) SEM micrograph of the deposited film showing the distribution of LTA(Li)-Ag crystals on the quartz surface. The inset displays an enlargement of a selected area of the original SEM micrograph. e) Emission maxima of the hydrated and dehydrated PEI/LTA(Li)-Ag zeolite composite, during 10 hydration/dehydration cycles using 260 nm as excitation wavelength. f) Plot displaying the emission maxima profiles behavior of the PEI/LTA(Li)-Ag zeolite composite after 10 hydration/dehydration cycles. Please click here to view a larger version of this figure.
Discussion
A simple device to demonstrate the proof of concept of using LTA(Li)-Ag as a luminescence based humidity sensor was produced by spray coating the LTA(Li)-Ag powder suspended in a PEI solution onto a quartz plate. The PEI solution produces a polymer layer with homogenous thickness when the water is evaporated. The polymer-zeolite composite layer displays similar luminescent properties as that of the zeolite in powder form. The PEI/LTA(Li)-Ag zeolite composite displays the expected water-responsive luminescent properties, whose emission color changes upon variations in the water content present in the composite at relatively low humidity scale.
Replacing Na with Li ions in LTA zeolites (calculated exchange rate 33%) has a notable impact on the self-assembly and stabilization of luminescent silver clusters in the LTA(Li) scaffolds leading to unique optical properties. The EQE of LTA(Li)-Ag as compared to LTA(Na)-Ag samples is enhanced by more than one order of magnitude. Moreover, the emission colors displayed by the LTA(Li)-Ag samples have a water-dependence, providing a potential application of the samples as luminescence based humidity sensors.
We have thus demonstrated an easy method to fabricate a luminescent film-like humidity sensor through which changes in hydration levels can be visually monitored simply by using a UV lamp. The availability of the raw materials, the direct visualization of the color changes correlated with humidity content, the photo-stability of the films, and the relative ease of fabricating cost-effective devices make these luminescent materials potential candidates to compete with state-of-the-art humidity sensors based upon electrical responses. The procedure described in this report could also be applied and extended to different substrates, at different micro and macro scales, to make the sensor more flexible. Additionally, several critical steps during the fabrication of Ag-zeolites, which play an important role in determining the final optical properties of such materials, were discussed in this protocol. For instance, the pre-cleaning of the raw zeolite material leads to the removal of optical and chemical impurities, as well as to homogenous zeolite crystal size distribution. This is crucial for the incorporation of zeolites into functional devices. One limitation of the present methodology is the restriction on the use of thin film sensors beyond 75 °C. This is mainly due to the decomposition of the PEI polymer, rather than to the degradation of the LTA(Li)-Ag zeolites, which can withstand up to 500 °C. The use of heat-resistant polymers, such as polyvinyl alcohol, could expand the temperature range up to 200 °C. We expect that further investigations will be directed to the development of methodologies for the synthesis of nanostructured Ag-zeolite composites with (multi)functional properties and finally to the design of advanced sensor prototypes.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors gratefully acknowledge financial support from the Belgian Federal government (Belspo through the IAP VI/27 and IAP-7/05 programs), the European Union’s Seventh Framework Programme (FP7/2007-2013 under grant agreement no. 310651 SACS), the Flemish government in the form of long-term structural funding “Methusalem” grant METH/08/04 CASAS, the “Strategisch Initiatief Materialen” SoPPoM program, and the Fund for Scientific Research Flanders (FWO) grant G.0349.12. W.B. gratefully acknowledge the chemistry department of the KU Leuven for a FLOF-scholarship. The authors thank UOP Antwerp for the kind donation of zeolite samples and the mechanical workshop of the KU Leuven for helping with the design and construction of the heating/vacuum cell used in this study.
Materials
| LTA(Na) zeolite | UOP | Molsiv adsorbent 4A | |
| Silver nitrate | Sigma Aldrich | 209139 | ≥99,0% |
| Lithium nitrate | Sigma Aldrich | 62574 | ≥99,0%, calc. on dry substances |
| Polyethyleneimine solution | Sigma Aldrich | 3880 | ~50% H2O |
| Scanning electron microscope (SEM) | JEOL | JSM-6010LV | |
| Thermogravimetric analyzer | TA instruments | Q500 | |
| Spectrofluorimeter | Edinburgh instruments | FLS980-s | |
| Integrating sphere | Labsphere | 4P-GPS-033-SL |
References
- De Cremer, G., et al. Characterization of Fluorescence in Heat-Treated Silver-Exchanged Zeolites. J. Am. Chem. Soc. 131, 3049-3056 (2009).
- De Cremer, G., et al. Optical Encoding of Silver Zeolite Microcarriers. Adv. Mater. 22, 957-960 (2010).
- Coutino-Gonzalez, E., et al. X-ray Irradiation-Induced Formation of Luminescent Silver Clusters in Nanoporous Matrices. Chem. Commun. 50, 1350-1352 (2014).
- De Cremer, G., et al. In Situ Observation of the Emission Characteristics of Zeolite-Hosted Silver Species During Heat Treatment. ChemPhysChem. 11, 1627-1631 (2010).
- Coutino-Gonzalez, E., et al. Thermally Activated LTA(Li)-Ag Zeolites with Water-Responsive Photoluminescence Properties. J. Mater. Chem. C. 3, 11857-11867 (2015).
- Seifert, R., Kunzmann, A., Calzaferri, G. The Yellow Color of Silver-Containing Zeolite. A. Angew. Chem. Int. Ed. 37, 1522-1524 (1998).
- Seifert, R., Calzaferri, G. Colors of Ag+-Exchanged Zeolite A. J. Phys. Chem. A. 104, 7473-7483 (2000).
- Sazama, P., Jirglova, H., Dedecek, J. Ag-ZSM-5 Zeolite as High-Temperature Water-Vapor Sensor Material. Mat. Lett. 62, 4239-4241 (2008).
- Zheng, Y., Li, X., Dutta, P. K. Exploitation of Unique Properties of Zeolites in the Development of Gas Sensors. Sensors. 12, 5170-5194 (2012).
- Sun, T., Seff, K. Silver Clusters and Chemistry in Zeolites. Chem. Rev. 94, 857-870 (1994).
- Yu, Y., Ma, J. P., Dong, Y. B. Luminescent Humidity Sensors Based on Porous Ln3+-MOFs. Cryst. Eng. Comm. 14, 7157-7160 (2012).
- Qi, H., Mader, E., Liu, J. Unique Water Sensors Based on . Sensor. Actuat. B-Chem. 185, 225-230 (2013).
- Basabe-Desmonts, L., Reinhoudt, D. N., Crego-Calama, M. Design of Fluorescent Materials for Chemical Sensing. Chem. Soc. Rev. 36, 993-1017 (2007).
- Yamazoe, N., Shimzu, Y. Humidity Sensors – Principles and Applications. Sensor. Actuator. 10, 379-398 (1986).
- International Organization of Legal Metrology. . The Scale of Relative Humidity of Air Certified Against Saturated Salt Solutions. , (1996).
- Coutino-Gonzalez, E., et al. Determination and Optimization of the Luminescent External Quantum Efficiency of Silver-Clusters Zeolite Composites. J. Phys. Chem. C. 117, 6998-7004 (2013).
- Yahiro, H., et al. EPR Study on NO Introduced into Lithium Ion-Exchanged LTA Zeolites. Phys. Chem. Chem. Phys. 4, 4255-4259 (2002).
- Shelyakina, M. K., et al. Study of Zeolite Influence on Analytical Characteristics of Urea Biosensor Based on Ion-Selective Field-Effect Transistors. Nanoscale Res. Lett. 9, 124 (2014).

