Using the Sleeve Technique in a Mouse Model of Aortic Transplantation – An Instructional Video
Summary
We present an orthotopic aortic transplantation model using the sleeve technique in mice. It is a very rapid anastomosis method, which can be employed in studies of vascular disease.
Abstract
Orthotopic aortic transplantation using the sleeve technique reduces injury to the aorta with failure rate of only 10-20%. The time to anastomose the aorta in mice using the sleeve method was short and easy averaging 20 min, permitting studies of iso/allo grafts. The following article describes the aortic transplantation procedure used in our laboratory. The mice were anesthetized with a mixture of 1.5% volume isoflurane and 100% oxygen through a face mask. At this point, the segment of the aorta between the renal arteries and its bifurcation was separated from the vena cava, freely prepared and clampedat the proximal and distal segments with a single silk suture. Prior to the removal of the aorta, a saline solution containing heparin was injected into the inferior vena cava. Then the aorta was cut between the clamps and a saline heparin solution was used to flush the lumen. The sleeve technique with monofilament sutures was used in order to transplant the abdominal aorta in the orthotopic position.
Introduction
As pointed out in a previous study, great attention has been paid to murine aortic transplant models which allow discrimination between specific vascular responses caused by the graft itself and certain systemic factors connected with an arteriogenic environment1,2,3. The major factor that plays a crucial role here is the availability of knockout and transgenic mice. Their involvement in such a model offers the possibility of identifying and determining new pathophysiological pathways associated with the development of degenerative vascular disease, such as atherosclerosis and aneurysm formation4,5.
It is worth noting that during grafting an intrinsic ischemia/reperfusion injury to the vessels intended for the transplant may appear. Therefore, the occurrence of specific problems with graft integrity or an unexpected inflammatory reaction during the post-operative period cannot be excluded possibly precluding pathophysiological changes in degenerative vascular diseases3,4,5,6,7. Sleeve anastomosis is the alternative end-in-end method for arterial anastomosis of vessels with a diameter of less than one millimeter and has been applied successfully in renal and cardiac transplantation in rats which was subsequently adapted to aortic transplantation in mice by Dambrin et al.8,9,10,11.
Aortic damage using the sleeve transplantation technique is minimized with a very low technical failure rate, due to it lasting only 20 min on average. Our previous results have demonstrated excellent functional and structural properties of an isograft in vivo after transplantation using the sleeve technique1. Dambrin et al. describe that after a short learning curve the success rate was over 78%10. Complications such as thrombosis are rare, for example Engelbrecht et al. did not observe thrombosis using the sleeve technique in renal transplantation in the rat8.
The murine aortic transplant model with sleeve anastomoses is a rapid and easy tool to study iso/allograft reactions in the transplanted vessel. This video illustrates the aortic transplantation procedure carried out in our laboratory. This transplantation model may be useful in defining the underlying pathological mechanisms of vascular degenerative disease and may contribute to further evaluation of molecular and pharmacological interventions12.
Protocol
Procedures involving animal subjects were approved by the Institutional Animal Care and Use Committee (IACUC) at RWTH Aachen University, AZ 84-02.04.2012.A234.
NOTE: The procedure is demonstrated using adult male wild type mice with CD1 background. Keep the mice in a specialized laboratory unit before and after the surgery, assuring proper access to food, specialized veterinary control, and treatment. If the animals are purchased from outside, allow one-week acclimatization before performing surgery.
1. Preparation of the Donor
- Use sterile materials and instruments to maintain sterile conditions during surgery to avoid infections.
- Anesthetize each mouse with a mixture of 1.5% by volume isoflurane and 100% oxygen through a face mask. Lay the mouse on a platform in the supine position and tape all its legs to the operating table. Check its reflexes by pinching the hind feet to be sure that the mouse is sufficiently anesthetized. Place ophthalmic ointment on the eyes to prevent drying during the procedure.
- Remove all hair from the abdomen using a depilatory gel or use a shaver. Perform the operation under sterile conditions. Disinfect the abdomen with alternating scrubs of chlorhexidine and sterile water.
- Remove the donor aorta via a midline abdominal incision with scissors or scalpel. Retract the bowel manually to the right. Gently manually reflect the intestines over to the side using powder free gloves.
- Place the bowel on a piece of gauze wetted with saline to keep it moist.
- Dissect away the abdominal aorta very carefully from the surrounding tissue using a blunt dissection with tweezers.
- Separate the segment of the aorta between the renal arteries and its bifurcation from the vena cava with tweezers.
- Secure all the small branches of this segment very carefully using 11-0 monofilament single suture.
- Before removing the aorta, inject 0.5 milliliters (mL) of saline solution containing 50 U of heparin into the inferior vena cava.
- Let the donor animal exsanguinate after the segment of the aorta is removed.
- Rinse the graft fully with saline and then transfer it immediately to a container of ice-cold saline.
2. Preparation of the Recipient
- Anesthetize the recipient animal with a mixture of 1.5% by volume isoflurane and 100% oxygen through a face mask, then remove the hair and disinfect (Section 1). Make a mid-line incision from the xiphoid to the pelvis with a scalpel and retract the abdominal walls. Place ophthalmic ointment on the eyes to prevent dryness during the procedure.
- Wrap the bowel in saline solution moistened gauze and displace very gently to the animal's right.
- Dissect the infrarenal aorta free between the renal arteries proximally and the bifurcation distally with tweezers.
- Secure all the small branches of this segment very carefully using 11-0 monofilament single suture.
- Clamp the proximal and distal portions of the aorta with a 6-0 single silk suture.
- Divide the aorta in the middle between the clamps and irrigate the cut ends with heparinized saline to flush the lumen open.
- Place the graft in the orthotopic position with the feeding vessel end inserted into the receiving vessel followed by suturing with 11-0 monofilament taking care to avoid any torsion of the aorta by properly aligning the donor and recipient (Figure 1)10.
- Carefully release the ligatures after conducting an inspection of the anastomosis. Release the distal clamp first. This results in low pressure holding the walls together prior to releasing the proximal high-pressure side.
- Perfuse the graft immediately and check for a visible pulse. Gently remove the remnants of the silk. The optimal overlap length between donor and recipient aorta is 1-2 mm.
- Return the abdominal contents to the abdominal cavity and close the whole wound with a running 3-0 polyglycolic acid suture.
- Give the mouse buprenorphine (0.05 mg/kg body weight subcutaneously (SC)) before terminating anesthesia.
- Do not leave an animal unattended until it is fully conscious. Manage pain therapy with buprenophine 0.05 mg/kg body weight administered SC three times a day for three days after the operation as approved by the institutional oversight body.
- For tissue harvesting, anesthetize the recipient mice as described above and flush the vessels with phosphate-buffered saline (PBS) followed by 4% formaldehyde/PBS, (pH = 7.4) by cardiac puncture. Remove the grafts gently. After overnight fixation in 4% formaldehyde/PBS, process specimens further and embed in paraffin.
Representative Results
The mice recovered from anesthesia within 15-30 min with no observed physical impairment, although there was an elevated risk of thrombosis. Ultrasound analysis was used during postoperative follow up. The wild type mice used in the study exhibited no changes in the dimensions of their lumen. Accordingly, neither stenoses nor aneurysmal formations were observed. The transplant animals failed to exhibit plaque development of vessel walls (Figure 2).
Conventional staining procedures such as immunohistology can be used to determine the plaque, pattern of the smooth muscle cells, and accumulation of macrophages. In our study the histological and immunohistochemical staining was performed 6 weeks after grafting to test the integrity of the graft. Histological staining (Hematoxylin and eosin (HE) and immunohistochemical staining (Smooth Muscle Actin (SMA) and Macrophage (MAC2)) (Figure 3) showed us unchanged distribution patterns of smooth muscle cells, intact endothelial cell lining, and no accumulation of cells in the intima. These findings indicate that no significant lesion or cell activation was detected in the grafted vessels (Figure 3).
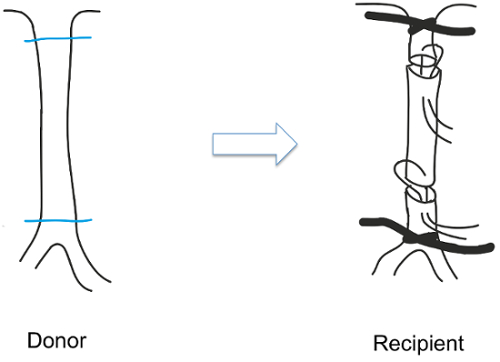
Figure 1: Sleeve technique. The abdominal aorta was transplanted using the sleeve technique. In this procedure, the donor's aorta was placed in the orthotopic position with superficial bites present in the feeding vessel. Please click here to view a larger version of this figure.
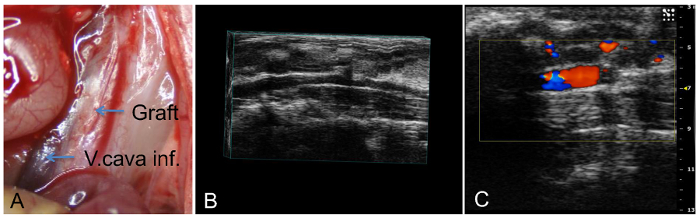
Figure 2: Intraoperative and ultrasound images. Examples of the intraoperative (necropsy) view of the grafts 6 weeks after transplantation (A), its three-dimensional ultrasound (B), and B-mode view (C). The postoperative follow up was conducted using ultrasound. The pictures show the patency of the graft with no change in lumen dimensions. Additionally, no stenoses or aneurysmal formations were observed. Please click here to view a larger version of this figure.
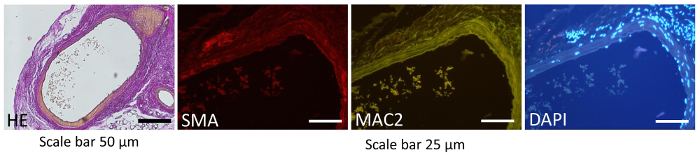
Figure 3: Histological and immunohistochemistry images. The histological and immunohistochemistry representative images of transplanted animals at 6 weeks after transplantation. No significant lesions were observed in transplanted aortas by histology (Hematoxylin and eosin, HE, 100X magnification, Scale bar = 50 µm), immunohistochemical staining (Smooth Muscle Actin SMA (red), or Macrophage MAC2 (green), 200X magnification, Scale bar = 25 µm). Nuclei were counter-stained by DAPI (blue). Please click here to view a larger version of this figure.
Discussion
Prior to this study various other transplantation models in mice were thoroughly analysed3,6,7,10,13,14. The model of aortic transplantation using the sleeve technique with modifications by Dambrin et al. was selected as it matched our criteria and showed a high reliability of the sleeve anastomosis in comparison to conventional end-to-end suture methods1,10.
This technique is favorable in many ways with cross-clamp time considerably reduced minimizing the damage to the aorta during surgery. A low incidence of thrombosis was observed in addition to avoiding a potential mismatch in vessel caliber between the donor and the recipient. The above observations make this technique highly suitable for investigating vascular disease in aortic transplants in mice.
In a follow-up study in which an ultrasound was performed 8 weeks after transplantation, no significant changes were detected. This confirmed assumptions that any damage to the aorta during surgery would be minimal1.
The grafting procedure presented in this article guarantees no impairment both in the graft integrity and its function. Therefore, it can be concluded that this experimental transplantation model may serve as a valuable tool for future molecular and pharmacological investigations of degenerative vessel disease in genetically engineered mice.
We believe that the video guide can function as teaching material illustrating the use of this simple arterio-arterial model and that it will contribute to further fruitful debate on many important issues in vascular pathologies. This very rapid anastomosis method may be used to study vascular disease in genetically engineered mice. It may also be used as a modification in the aneurysm model combined with transplantation.
There are critical points during the procedure. The placing of the suture itself is the most critical step. The surgeon has to take care to avoid any torsion of the aorta by proper alignment of donor and recipient. The clamps are carefully removed after inspection of the anastomosis. The distal clamp should always be released first resulting in low pressure holding the walls together prior to the proximal high-pressure side release. The consequence of not properly following the sequence of release would be bleeding.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Roma Wieczorek and Peter Kurdybacha for their excellent editing assistance, and Leon Decker and Uli Heuter for their excellent technical assistance.
Materials
| Halsey Needle Holder | Fine Science Tools | 12001-13 | |
| Dumont #5-45 Forceps | Fine Science Tools | 11251-35 | |
| Dumont #5 Forceps – | Fine Science Tools | 11254-20 | |
| Lexer-Baby Scissors | Fine Science Tools | 14079-10 | |
| Castroviejo Micro Needle Holder | Fine Science Tools | 12060-01 | |
| Vannas Scissors | Aesculap, Germany | Typ OC498R | |
| Castroviejo Suture Forceps | Geuder, Germany | 19015 | |
| 6-0 silk black (Silk) | Deknatel, Research Triangle Park NC, USA | 18020-60- FST | |
| 11-0 monofilament (Ethilon) | Ethicon, Norderstedt, Germany | EH7438G | |
| 3-0 polyglycolic acid suture (Serafit) | Serag-Wiessner, Naila, Germany | 60203214 | |
| Isofluorane | Any genericon | ||
| Heparin | Any genericon | ||
| 0.9% saline | Any genericon | ||
| Buprenorphine | Any genericon | ||
| Bepanthene eye and nose cream | Bayer, Germany | ||
| Microscop | Zeiss | Opmi MDO/S5 | |
| Vaporiser | Eickemeyer | TEC3 | |
| Ultrasound | Vevo, Canada | 770,2100 |
References
- Rowinska, Z., et al. Non- invasive in vivo analysis of a murine aortic graft using high resolution ultrasound microimaging. Eur J Radiol. 81 (2), 244-249 (2012).
- Daugherty, A. Mouse models of atherosclerosis. Am J Med Sci. 323 (1), 3-10 (2002).
- Xu, Q. Mouse models of arteriosclerosis: from arterial injuries to vascular grafts. Am J Pathol. 165 (1), 1-10 (2004).
- Daugherty, A., Cassis, L. A. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc .Biol. 24 (3), 429-434 (2004).
- Zernecke, A., Shagdarsuren, E., Weber, C. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. 28 (11), 1897-1908 (2008).
- Chereshnev, I., et al. Mouse model of heterotopic aortic arch transplantation. J Surg Res. 111 (2), 171-176 (2003).
- Koulack, J., McAlister, V. C., Giacomantonio, C. A., Bitter-Suermann, H., MacDonald, A. S., Lee, T. D. Development of a mouse aortic transplant model of chronic rejection. Microsurgery. 16 (2), 110-113 (1995).
- Engelbrecht, G., Kahn, D., Duminy, F., Hickman, R. New rapid technique for renal transplantation in the rat. Microsurgery. 13 (6), 340-344 (1992).
- Baxter, K., Hao, P. M., Howden, B. O., Saunder, A., Jablonski, P. Modified technique of abdominal heart transplantation in the rat. J Heart Lung Transplant. 17 (11), 1057-1064 (1998).
- Dambrin, C., Calise, D., Pieraggi, M. T., Thiers, J. C., Thomsen, M. Orthotopic aortic transplantation in mice: a new model of allograft arteriosclerosis. J Heart Lung Transplant. 18 (10), 946-951 (1999).
- Siemionow, M. Histopathology of microarterial anastomoses: end-to-end versus end-in-end (sleeve) technique. J Hand Surg Am. 15, 619-625 (1990).
- Charo, I. F., Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 354 (6), 610-621 (2006).
- Sun, H., et al. Improved surgical technique for the establishment of a murine model of aortic transplantation. Microsurgery. 18 (6), 368-371 (1998).
- Guo, L., Agarwal, A., George, J. F. Orthotopic aortic transplantation in mice for the study of vascular disease. J Vis Exp. (69), e4338 (2012).

