Using Scaffold Liposomes to Reconstitute Lipid-proximal Protein-protein Interactions In Vitro
Summary
This paper describes a method for assessing the interactions and assemblies of integral membrane proteins in vitro with various partner factors in a lipid-proximal environment.
Abstract
Studies of integral membrane proteins in vitro are frequently complicated by the presence of a hydrophobic transmembrane domain. Further complicating these studies, reincorporation of detergent-solubilized membrane proteins into liposomes is a stochastic process where protein topology is impossible to enforce. This paper offers an alternative method to these challenging techniques that utilizes a liposome-based scaffold. Protein solubility is enhanced by deletion of the transmembrane domain, and these amino acids are replaced with a tethering moiety, such as a His-tag. This tether interacts with an anchoring group (Ni2+ coordinated by nitrilotriacetic acid (NTA(Ni2+)) for His-tagged proteins), which enforces a uniform protein topology at the surface of the liposome. An example is presented wherein the interaction between Dynamin-related protein 1 (Drp1) with an integral membrane protein, Mitochondrial Fission Factor (Mff), was investigated using this scaffold liposome method. In this work, we have demonstrated the ability of Mff to efficiently recruit soluble Drp1 to the surface of liposomes, which stimulated its GTPase activity. Moreover, Drp1 was able to tubulate the Mff-decorated lipid template in the presence of specific lipids. This example demonstrates the effectiveness of scaffold liposomes using structural and functional assays and highlights the role of Mff in regulating Drp1 activity.
Introduction
Studying membrane-proximal protein-protein interactions is a challenging endeavor due to difficulty in recapitulating the native environment of the integral membrane proteins involved1. This is due to the necessity of detergent solubilization and the inconsistent orientation of proteins in proteoliposomes. In order to avoid these issues, we have employed a strategy whereby soluble domains of integral membrane proteins are expressed as His-tag fusion proteins, and these soluble fragments are anchored to scaffold liposomes via interactions with NTA(Ni2+) headgroups at the lipid surface. Using these scaffolds, lipid-proximal protein interactions can be investigated over a range of lipid and protein compositions.
We have effectively applied this method to investigate the critical protein-protein interactions that govern assembly of the mitochondrial fission complex and examine lipid interactions that modulate this process2. During mitochondrial fission, a conserved membrane remodeling protein, called Dynamin-related protein 1 (Drp1)3, is recruited to the surface of the Outer Mitochondrial Membrane (OMM) in response to cellular signals that regulate energy homeostasis, apoptotic signaling, and several other integral mitochondrial processes. This large, cytosolic GTPase is recruited to the surface of mitochondria through interactions with integral OMM proteins4–8. The role of one such protein, Mitochondrial Fission Factor (Mff), has been difficult to elucidate due to an apparent weak interaction with Drp1 in vitro. Nevertheless, genetic studies have clearly demonstrated that Mff is essential for successful mitochondrial fission7,8. The method described in this manuscript was able to overcome previous shortcomings by introducing simultaneous lipid interactions that promote Drp1-Mff interactions. Overall, this novel assay revealed fundamental interactions guiding assembly of the mitochondrial fission complex and provided a new stage for ongoing structural and functional studies of this essential molecular machine.
To date, examination of interactions between Drp1 and Mff have been complicated by the inherent flexibility of Mff9, the heterogeneity of Drp1 polymers2,10, and the difficulty in purifying and reconstituting full-length Mff with an intact transmembrane domain11. We addressed these challenges by using NTA(Ni2+) scaffold liposomes to reconstitute His-tagged Mff lacking its transmembrane domain(MffΔTM-His6). This strategy was advantageous because MffΔTM was extremely soluble when over-expressed in E. coli, and this isolated protein was easily reconstituted on scaffold liposomes. When tethered to these lipid templates, Mff assumed an identical, outward facing orientation on the surface of the membrane. In addition to these advantages, mitochondrial lipids, such as cardiolipin, were added to stabilize Mff folding and association with the membrane11. Cardiolipin also interacts with the variable domain of Drp12,12 which may stabilize this disordered region and facilitate assembly of the fission machinery.
This robust method is widely applicable for future studies that seek to evaluate membrane-proximal protein interactions. Through the use of additional tethering/affinity interactions, the sophistication of these membrane reconstitution studies can be enhanced to mimic additional complexity found at the surface of membranes within cells. At the same time, lipid compositions can be modified to more accurately mimic the native environments of these macromolecular complexes. In summary, this method provides a means to examine the relative contributions of proteins and lipids in shaping membrane morphologies to during critical cellular processes.
Protocol
1. Scaffold Liposome Preparation
NOTE: Ideally, initial experiments should use a relatively simple and featureless scaffold (comprised of DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine or PC) and DGS-NTA(Ni2+) (1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl](nickel salt)). Building off of these experiments, lipid charge, flexibility, and curvature can be introduced as individual factors with the potential to alter membrane-proximal interactions. These changes can be achieved by adding defined amounts of specific lipid constituents, including phosphatidylserine or cardiolipin (CL), phosphatidylethanolamine (DOPE or PE), or galactosyl(β) ceramide.
- Combine lipids dissolved in chloroform in a clean glass test tube. Evaporate the solvent with dry nitrogen gas while rotating the tube to form a thin lipid film. Remove residual solvent with a centrifugal evaporator for 1 h at 37 °C.
NOTE: Various liposome formulations are used in the protocols described below: scaffold liposomes (3.3 mol% DGS-NTA(Ni2+) / 96.7 mol% DOPC), scaffold liposomes with cardiolipin (3.3 mol% DGS-NTA(Ni2+) / 10 mol% cardiolipin / 86.7 mol% DOPC), flexible scaffold liposomes with cardiolipin (3.3 mol% DGS-NTA(Ni2+) / 10 mol% cardiolipin / 35 mol% DOPE / 51.7 mol% DOPC), and enriched scaffold liposomes (10 mol% DGS-NTA(Ni2+) / 15 mol% cardiolipin / 35 mol% DOPE / 40 mol% DOPC). - Add Buffer A (25 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 150 mM KCl, pH adjusted to 7.5 with KOH) preheated to 37 °C such that the final lipid concentration is 1 – 2 mM. Incubate 30 min at 37 °C with occasional vortexing to fully resuspend the lipid mixture (Figure 1a).
- Transfer to a plastic test tube, place the tube in liquid nitrogen until completely frozen (roughly 30 s), and place in a 37 °C water bath until fully thawed (roughly 1 – 2 min). Repeat for a total of 4 freeze-thaw cycles (Figure 1b).
- Prepare a lipid extruder by soaking 4 filter supports and a polycarbonate filter in buffer and assembling the extruder according to manufacturer instructions. Extrude the lipid solution through the filter 21 times. Use gentle, constant pressure to ensure a homogenous size distribution (Figure 1c).
NOTE: For all experiments described in this protocol, a 1.0 µm polycarbonate filter was used for extrusion. Drp1 interaction with anionic lipids can be observed with a variety of liposome diameters ranging from 50 nm to 400 nm12 or larger13. Hence, the filter size of 1 µm was chosen to be ideal for both GTPase activity and for electron microscopy. If other liposome diameters are desired, preparation of giant unilamellar vesicles14,15 (GUVs) or small unilamellar vesicles16 (SUVs) can be used. Dynamic light scattering can be used to assess liposome size heterogeneity13. - Store extruded liposomes at 4 °C and discard after 3 – 5 d.
2. Use of Scaffold Liposomes for Protein Binding Analysis
- Sample Preparation
- Incubate His-tagged MffΔTM (5 µM final) with scaffold liposomes (40 mol% PC / 35 mol% PE / 15 mol% CL / 10 mol% DGS-NTA(Ni2+); 50 µM final) for at least 15 min at RT in Buffer A + BME (25 mM HEPES, 150 mM KCl, 10 mM β-mercaptoethanol (BME), pH adjusted to 7.5 with KOH). For an Mff-free control, incubate liposomes with a his-tagged control protein (such as GFP) to bind and shield exposed NTA(Ni2+).
NOTE: MffΔTM was expressed and purified as described in a previous study2. GFP was purified in a similar manner, but the ion-exchange step was omitted. BME was required for these experiments because Drp1 is sensitive to oxidation, which can alter its activity and assembly properties. - Add Drp1 (2 µM final) and incubate for 1 h at RT.
NOTE: Drp1 was expressed and purified as described in a previous study2. After incubation with Drp1, the effect of nucleotide binding on membrane deformation can be investigated by incubating one additional hour with 2 mM MgCl2 and either 1 mM GTP, 1 mM GMP-PCP, or Buffer A + BME.
- Incubate His-tagged MffΔTM (5 µM final) with scaffold liposomes (40 mol% PC / 35 mol% PE / 15 mol% CL / 10 mol% DGS-NTA(Ni2+); 50 µM final) for at least 15 min at RT in Buffer A + BME (25 mM HEPES, 150 mM KCl, 10 mM β-mercaptoethanol (BME), pH adjusted to 7.5 with KOH). For an Mff-free control, incubate liposomes with a his-tagged control protein (such as GFP) to bind and shield exposed NTA(Ni2+).
- Negative Stain Transmission Electron Microscopy (EM) Analysis
- Transfer 5 µL of sample to a sheet of laboratory film, and lay a carbon-coated Cu/Rh grid on the sample. Incubate the grid 1 min on the sample, blot away excess liquid on filter paper, and transfer to a drop of 2% uranyl acetate. Incubate 1 min, blot excess stain on filter paper, and transfer to a grid box. Store under vacuum O/N to ensure full desiccation.
- Image samples using a transmission electron microscope at 18,500 – 30,000X magnification to observe ultrastructural changes in protein and liposome morphologies17.
Note: Ultrastructural changes can be quantified using image analysis software, such as ImageJ13 (http://imagej.nih.gov/ij/). Protein decoration can be measured when compared with naked lipid templates. Additionally, the diameters of tubular segments can be measured from the outermost portion of the assemblies13. A more detailed analysis can be performed using cryo-electron microscopy17. This method can be used to image native protein-lipid assemblies in solvent without the use of heavy metal stains that coat the sample. In this way, detailed structural features not apparent in negative stain, including changes in the underlying lipid morphology, can be examined and quantified.
3. Use of Scaffold Liposomes for Enzymatic Assay
Note: A colorimetric GTPase assay18 was used to measure phosphate liberation via GTP hydrolysis. Alternative GTPase assays are available19 and can be implemented as needed.
- Incubate His-tagged MffΔTM (Mff), Fis1ΔTM(Fis1), or GFP (5 µM final for all) with scaffold liposomes (150 µM final) for 15 min at RT in Buffer A + BME (volume = 30 µL). Add Drp1 (500 nM final) and incubate an additional 15 min at RT (volume = 80 µL).
NOTE: Fis1 was purified in a similar manner to Mff2, but the ion-exchange chromatography step was omitted. The purpose of His-tagged GFP is to shield the NTA(Ni2+) headgroups and prevent nonspecific charge interactions with other proteins. If no effect is observed in the absence of GFP, then this control may not be required. Alternative blocking proteins (of comparable size to the protein of interest) can be used as well, but GFP allows for direct visualization of the interactions with scaffold liposomes. - Transfer tubes to a thermocycler set to 37 °C, and initiate reactions by addition of GTP and MgCl2 (1 mM and 2 mM final, respectively; volume = 120 µL).
- At desired time points (i.e. T = 5, 10, 20, 40, 60 min), transfer 20 µL of reaction to wells of a microtiter plate containing 5 µL of 0.5 M EDTA to chelate Mg2+ and stop the reaction.
- Prepare a set of phosphate standards by diluting KH2PO4 in Buffer A + BME to calibrate results. A useful set of standards is 100, 80, 60, 40, 20, 10, 5, and 0 µM. Add 20 µL of each to wells containing 5 µL of 0.5 M EDTA.
- Add 150 µL of Malachite green reagent (1 mM malachite green carbinol, 10 mM ammonium molybdate tetrahydrate, and 1 N HCl) to each well, and read OD650 5 min after addition.
NOTE: GTP is acid labile, and will hydrolyze in the presence of malachite green reagent. Ensure that the time between adding malachite reagent and reading is constant to ensure reproducible results. - Generate a standard curve by plotting OD650 of the standards as a function of phosphate concentration. Use linear regression to determine the relationship between OD650 and phosphate concentration in a sample.
- Using the linear regression, convert the OD650 of the protein reaction samples to µM phosphate. Determine the rate of phosphate generation for each reaction mixture by plotting phosphate concentration as a function of time, and convert to kcat by dividing the rate by the Drp1 concentration (0.5 µM).
NOTE: Only the initial linear rate should be used to determine the rate of phosphate generation, and a minimum of 3 data points must be used. If the rate of reaction is sufficiently rapid that the first three data points are not linear (i.e. the r2 of the linear fit is less than 0.9) a significantly shorter time course with at least 3 time points should be performed.
Representative Results
While the interaction between Drp1 and Mff has been demonstrated to be important for mitochondrial fission, this interaction has been difficult to recapitulate in vitro. Our goal was to better emulate the cellular environment wherein Drp1 and Mff interact. To this end, liposomes containing limiting concentrations of NTA(Ni2+) headgroups were prepared by rehydrating a lipid film as described above. The lipid solution initially consists of unilamellar and multilamellar vesicles of heterogeneous diameters as evidenced by the opacity of the solution (Figure 1a). This opacity is reduced by freeze-thawing (Figure 1b), which reduces the prevalence of multilamellar vesicles. The liposome diameters are further homogenized by extrusion through a polycarbonate filter, which results in a clear solution (Figure 1c).
In previous studies, we found that Drp1 was able to assemble on Mff-decorated scaffold liposomes, and membrane tubulation was observed when flexible membranes were employed2. Building on these findings, we utilized a new template composed of PC, PE, Ni, and CL (called Enriched Scaffold Liposomes or ESL) to promote ordered assembly of a polymeric Drp1-Mff complex capable of inducing membrane deformation. Specifically, increased NTA(Ni2+) and cardiolipin lipids were utilized (10 mol% and 15 mol% respectively) for this application. Then, GFP or Mff was tethered to ESL templates in the presence and absence of Drp1 (Figure 2), and the ability of Drp1 to remodel membranes was qualitatively assessed. In the absence of Drp1, neither Mff nor GFP resulted in membrane deformation (Figure 3a, b), and similarly in the case of GFP-decorated ESL, only featureless liposomes were observed (Figure 3c). However, when Drp1 was added to Mff-decorated ESL templates, remodeling of the liposomes was evident (Figure 3d).
While macromolecular complex formation clearly demonstrates an interaction between Drp1 and Mff, this qualitative analysis alone is incapable of determining the functional effects of such an interaction. Therefore, we utilized a malachite green phosphate generation assay18 to assess alterations in the catalytic activity of Drp1 in response to interaction with Mff. As described previously2, we initially utilized a simple scaffold liposome (SL; 3.3 mol% DGS-NTA(Ni2+), 96.7 mol% DOPC) to investigate the effect of Mff alone on Drp1 structure and function. Nonspecific interaction of Drp1 with NTA(Ni2+) has previously been described20, so SL was initially designed to contain low concentrations of NTA(Ni2+) to avoid nonspecific activity stimulation of Drp1. With the larger amounts of NTA(Ni2+) in ESL, the use of His-tagged GFP as a control was found to be critical to shield the Ni2+ and prevent non-specific Drp1 interactions. After decoration of SL liposomes by Mff or GFP (as illustrated in Figure 2), the extent of self-assembly can be assessed by measuring the GTPase activity of Drp1. In the absence of liposomes, Drp1 has a relatively low basal GTPase activity, which is slightly enhanced by addition of SL. Decoration of these scaffold liposomes with Mff enhanced GTPase activity (Figure 4a, 1.8 fold). Conversely, when the exposed NTA(Ni2+) headgroups were blocked with His-tagged GFP, this augmented GTPase activity was ablated. We also tested the role of Fis1, an OMM protein that has been suggested to have a role in mitochondrial fission21,22, though this has been challenged in recent studies7,23. Tethering of Fis1 lacking its transmembrane domain to SL also failed to elicit a stimulation of Drp1's GTPase activity (Figure 4a).
We then utilized a slightly more complex lipid scaffold containing a small amount of cardiolipin (SL/CL: SL with 10 mol% cardiolipin replacing DOPC) to determine the role of this mitochondrial lipid in the interaction of Drp1 and Mff. This moderate concentration of cardiolipin was specifically chosen to limit the stimulation of Drp1 by cardiolipin as described previously10. Similar to SL, addition of SL/CL to Drp1 resulted in a slight stimulation of GTPase activity that was reversed by tethering His-tagged Fis1 or GFP to the liposomes. A synergy between Mff and cardiolipin was observed as the GTPase activity of Drp1 was stimulated 2.6 fold when it was incubated with Mff-decorated SL/CL (Figure 4b).
Membrane fluidity and the ability of Drp1 to remodel lipid bilayers have been proposed to enhance its GTPase activity. Therefore, we sought to examine the effect of membrane fluidity/flexibility using a flexible scaffold liposome. This was achieved by replacing 35 mol% of DOPC in SL/CL with DOPE (SL/PE/CL), which has previously been shown to allow for Drp1-mediated membrane remodeling10. Addition of undecorated SL/PE/CL scaffold liposomes to Drp1 slightly enhances Drp1 GTPase activity, and decoration of these liposomes with GFP eliminates this effect. When SL/PE/CL templates were decorated with Mff, Drp1 activity was enhanced (Figure 4c, 2.4 fold). As we have previously shown, the ability of Drp1 to remodel liposomes into lipid tubules was enhanced by the addition of PE to the scaffold liposomes. Interestingly, this improved tubulation leading to the formation of a helical Drp1 polymer did not result in any greater stimulation when compared to liposomes that Drp1 was unable to remodel2.
Using these adaptable lipid templates, Mff and Drp1 were found to interact in a more native environment in vitro. This technique has enabled us to control the relative abundance of Drp1, Mff (through NTA(Ni2+) concentration), and specific lipids (cardiolipin and PE specifically) that appeared to regulate the assembly of this macromolecular complex. As we have demonstrated, this method can be utilized to visualize the membrane remodeling of Mff-recruited Drp1 by electron microscopy, and to determine the effects of Drp1 assembly on its catalytic activity using GTPase activity assay.
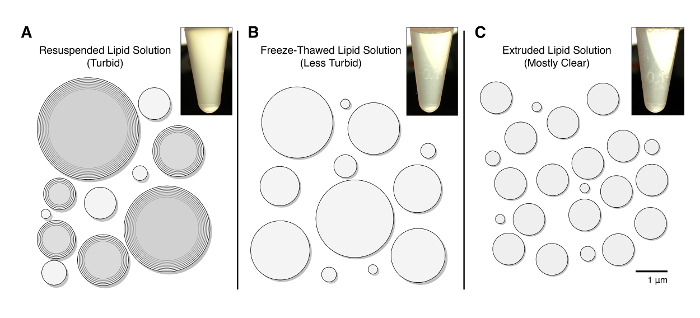
Figure 1: Lipid Preparation Schematic. (a) Upon resuspension, liposomes of diverse sizes form and consist of unilamellar and multilamellar vesicles, which results in an opaque solution (inset). (b) Freeze-thawing the solution results in a more unilamellar population of liposomes, which are still heterogeneous in diameter. Freeze-thawing clarifies the solution (inset). (c) Extrusion of the lipid solution homogenizes the liposome diameter (1.0 µm in this example), and results in a clear solution (inset). Please click here to view a larger version of this figure.
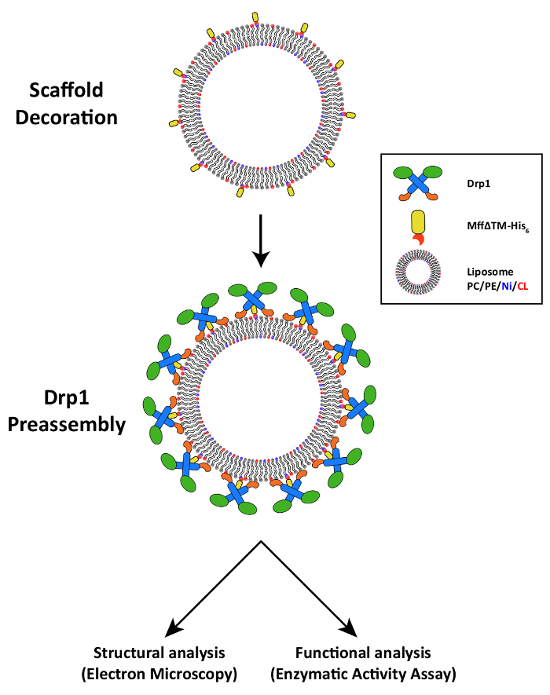
Figure 2: Methods to Assess Protein assembly. A schematic depicting partner protein assembly on scaffold liposomes is presented. His-tagged partner proteins or GFP are incubated with scaffold liposomes, and then Drp1 is incubated with decorated or undecorated liposomes. These Drp1-preassembled liposomes can then be analyzed by structural methods (electron microscopy) and functional assays (GTPase assay). Please click here to view a larger version of this figure.
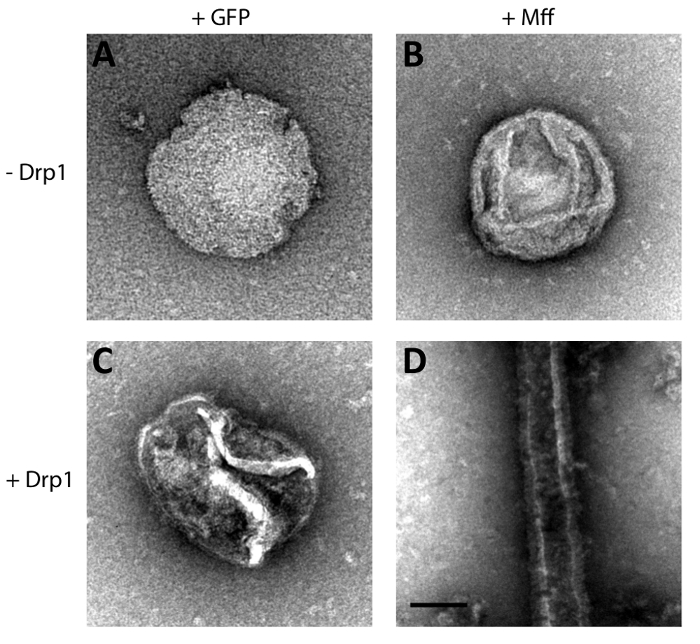
Figure 3: Structural Assessment of Drp1 Recruitment. Negative stain transmission micrographs of GFP or Mff decorated liposomes alone (A, B, respectively) or incubated with Drp1 (C, D, respectively). Scale bar = 100 nm. Please click here to view a larger version of this figure.
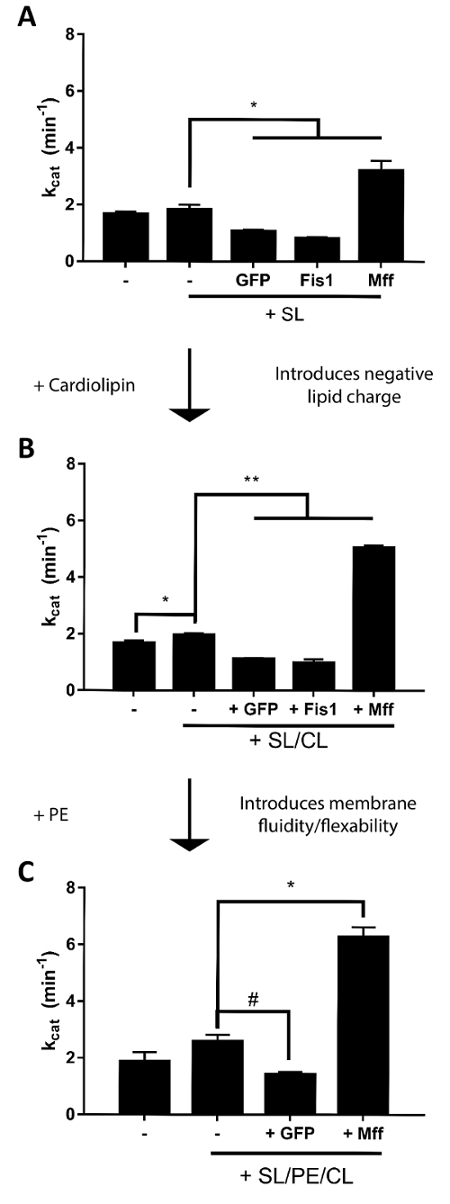
Figure 4: Scaffold Liposome Enzymatic Assay. (A – C) The generation of phosphate over time was measured (inset), and the kcat was determined. This method was applied to SL-tethered proteins (A), SL/CL-tethered proteins (B), or SL/PE/CL-tethered proteins (C). #: p <0.05, *: p <0.0001, **: p <0.000001 as determined by unpaired student's T-test. All error bars represent standard deviation from 3 independent samples. Please click here to view a larger version of this figure.
Discussion
This protocol offers a method for investigating protein-protein interactions involving integral membrane proteins. By utilizing a modular liposome scaffold, investigators are capable of assessing the activity of one or more proteins in a lipid-proximal environment. Previous studies have demonstrated a similar method for receptor enzymes of the plasma membrane24–26. We have expanded this method to incorporate lipid cofactors and explore interactions between proteins that make up the mechanoenzymatic core of the mitochondrial fission machinery.
For the model system presented above, we found that Mff-decorated SL enhanced Drp1 self-assembly. Moreover, we now show that, Mff-decorated ESL templates were efficiently remodeled by wild-type Drp1 to form extended tubular structures. We also assessed the roles of various mitochondrial lipids, including the negatively charged cardiolipin and the conical lipid PE. Cardiolipin synergizes with Mff to further enhance Drp1 self-assembly, while the membrane flexibility and fluidity imparted by PE enhances membrane tubulation but does not further augment Mff-induced stimulation.
To assess ultrastructural changes in membrane morphologies, EM analyses were required. Drp1 GTPase activity was elevated through clustering and assembly of filamentous polymers that did not reshape the liposomes to any great extent2. However, membrane deformation was observed when the more mitochondria-like SL/PE/CL template was used. Interestingly, the enzyme activity was not enhanced. Therefore, the EM studies were essential in identifying key differences that would otherwise be missed using the functional assay.
While this technique is powerful for exploring the function and interaction of soluble proteins and soluble protein domains, these lipid scaffolds cannot account for the role of transmembrane domains. This is an important consideration because the transmembrane domain can effect dynamic protein processes such as self-assembly27 and lateral diffusion28–30 in lipid bilayers. If these factors are critical for evaluating protein interactions at the membrane surface, then traditional lipid reconstitution experiments with detergent would be favored. Alternative tethers may also be explored to control the recruitment and mobility of the membrane anchored proteins.
In addition to using His-tagged proteins with NTA(Ni2+) lipid anchors, other tethers such as biotin-conjugated31 or reactive group-conjugated lipids can be utilized. These covalent modifications would more stably trap proteins at the lipid surface, but mobility and exchange of these factors would likely be diminished. As such, the tether should be carefully considered in the context of the protein complexes being studied. When considering the use of this method, the mode of tethering proteins to lipid templates have the potential to influence certain assays. For instance, the His-tag tethering to NTA(Ni2+) method may be more appropriate for in situ assays rather than separation experiments, especially in the case of transient protein-protein interactions. This is clearly demonstrated in Figure 3 by the discrepancy between the in situ negative stain electron microscopy and the sedimentation assay.
In the future, a combination of two or more of these anchor lipids with distinct target headgroups could be implemented to allow for recruitment of multiple proteins to a scaffold template without competition for a single lipid tether. Moreover, the relative abundance of each component can be managed by altering the lipid composition. Additional lipid cofactors, such as phosphoinositides, cardiolipin, and phosphatidylserine, can easily be introduced in these templates to assess the isolated impact of a variety of factors.
Overall, these lipid scaffolds represent a novel platform for investigating complex protein interactions near lipid membranes. These templates are easily generated and are simple to tailor to a range of diverse applications, including enzymatic assays, electron microscopy, or fluorescence imaging. In addition, the lipid composition can be formulated to resemble an organelle or membrane microdomain of interest to better recapitulate protein function at these specific regions. Using these techniques, biochemists can probe the complex interactions of membrane bound and membrane associated proteins with their partners and their environment.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the funding received from the American Heart Association (SDG12SDG9130039).
Materials
| Phosphatidylcholine (DOPC) | Avanti Polar Lipids | 850375 | |
| Phosphatidylethanolamine (DOPE) | Avanti Polar Lipids | 850725 | |
| DGS-NTA(Ni2+) | Avanti Polar Lipids | 790404 | |
| Bovine Heart Cardiolipin (CL) | Avanti Polar Lipids | 840012 | |
| Chloroform | Acros Organics | 268320010 | |
| Liposome Extruder | Avanti Polar Lipids | 610023 | |
| Cu/Rh Negative Stain Grids | Ted Pella | 79712 | |
| Microfuge Tube | Beckman | 357448 | |
| GTP | Jena Biosciences | NU-1012 | |
| GMP-PCP | Sigma Aldrich | M3509 | |
| Microtiter Plate strips | Thermo Scientific | 469949 | |
| EDTA | Acros Organics | 40993-0010 | |
| Instant Blue Coomassie Dye | Expedeon | ISB1L | |
| HEPES | Fisher Scientific | BP310 | |
| BME | Sigma Aldrich | M6250 | |
| KCL | Fisher Scientific | P330 | |
| KOH | Fisher Scientific | P250 | |
| Magnesium Chloride | Acros Organics | 223211000 | |
| 4-20% SDS-PAGE Gel | Bio Rad | 456-1096 | |
| 4x Laemmli Loading Dye | Bio Rad | 161-0747 | |
| HCL | Fisher Scientific | A144S | |
| Malachite Green Carbinol | Sigma Aldrich | 229105 | |
| Ammonium Molybdate Tetrahydrate | Sigma Aldrich | A7302 | |
| Laboratory Film | Parafilm | PM-996 | |
| Uranyl Acetate | Polysciences | 21447 | |
| Tecnai T12 100 keV Microscope | FEI | ||
| Optima MAX | Beckman | ||
| TLA-55 Rotor | Beckman | ||
| Refrigerated CentriVap Concentrator | Labconico | ||
| Mastercycler Pro Thermocycler | Eppendorf | ||
| VersaMax Microplate reader | Molecular Devices |
References
- Seddon, A. M., Curnow, P., Booth, P. J. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta (BBA) – Biomembranes. 1666 (1-2), 105-117 (2004).
- Clinton, R. W., Francy, C. A., Ramachandran, R., Qi, X., Mears, J. A. Dynamin-related Protein 1 Oligomerization in Solution Impairs Functional Interactions with Membrane-anchored Mitochondrial Fission Factor. J Biol Chem. 291 (1), 478-492 (2016).
- Chan, D. C. Mitochondrial Fusion and Fission in Mammals. Annual Review of Cell and Dev Biol. 22 (1), 79-99 (2006).
- Bui, H. T., Shaw, J. M. Dynamin Assembly Strategies and Adaptor Proteins in Mitochondrial Fission. Curr Biol. 23 (19), R891-R899 (2013).
- Elgass, K., Pakay, J., Ryan, M. T., Palmer, C. S. Recent advances into the understanding of mitochondrial fission. Biochim Biophys Acta (BBA) – Mol Cell Res. 1833 (1), 150-161 (2013).
- Losón, O. C., Song, Z., Chen, H., Chan, D. C. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 24 (5), 659-667 (2013).
- Otera, H., Wang, C., et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 191 (6), 1141-1158 (2010).
- Gandre-Babbe, S., van der Bliek, A. M. The Novel Tail-anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells. Mol Biol Cell. 19 (6), 2402-2412 (2008).
- Koirala, S., Guo, Q., et al. Interchangeable adaptors regulate mitochondrial dynamin assembly for membrane scission. Proc Natl Acad Sci. 110 (15), E1342-E1351 (2013).
- Macdonald, P. J., Stepanyants, N., et al. A dimeric equilibrium intermediate nucleates Drp1 reassembly on mitochondrial membranes for fission. Mol Biol Cell. 25 (12), 1905-1915 (2014).
- Macdonald, P. J., Francy, C. A., et al. Distinct Splice Variants of Dynamin-related Protein 1 Differentially Utilize Mitochondrial Fission Factor as an Effector of Cooperative GTPase Activity. J Biol Chem. 291 (1), 493-507 (2016).
- Bustillo-Zabalbeitia, I., Montessuit, S., Raemy, E., Basañez, G., Terrones, O., Martinou, J. -. C. Specific Interaction with Cardiolipin Triggers Functional Activation of Dynamin-Related Protein 1. PLoS ONE. 9 (7), (2014).
- Francy, C. A., Alvarez, F. J. D., Zhou, L., Ramachandran, R., Mears, J. A. The Mechanoenzymatic Core of Dynamin-Related Protein 1 Comprises the Minimal Machinery Required for Membrane Constriction. J Biol Chem. 290 (18), 11692-11703 (2015).
- Walde, P., Cosentino, K., Engel, H., Stano, P. Giant Vesicles: Preparations and Applications. ChemBioChem. 11 (7), 848-865 (2010).
- Moscho, A., Orwar, O., Chiu, D. T., Modi, B. P., Zare, R. N. Rapid preparation of giant unilamellar vesicles. Proc Natl Acad Sci. 93 (21), 11443-11447 (1996).
- Klingler, J., Vargas, C., Fiedler, S., Keller, S. Preparation of ready-to-use small unilamellar phospholipid vesicles by ultrasonication with a beaker resonator. Anal Biochem. 477, 10-12 (2015).
- Mears, J. A., Hinshaw, J. E. Chapter 13 Visualization of Dynamins. Methods Cell Biol. 88, 237-256 (2008).
- Leonard, M., Doo Song, B., Ramachandran, R., Schmid, S. L. Robust Colorimetric Assays for Dynamin’s Basal and Stimulated GTPase Activities. Methods Enzymol. 404, 490-503 (2005).
- Ingerman, E., Perkins, E. M., et al. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 170 (7), 1021-1027 (2005).
- Fröhlich, C., Grabiger, S., et al. Structural insights into oligomerization and mitochondrial remodelling of dynamin 1-like protein. EMBO J. 32 (9), 1280-1292 (2013).
- James, D. I., Parone, P. A., Mattenberger, Y., Martinou, J. -. C. hFis1, a Novel Component of the Mammalian Mitochondrial Fission Machinery. J Biol Chem. 278 (38), 36373-36379 (2003).
- Yoon, Y., Krueger, E. W., Oswald, B. J., McNiven, M. A. The Mitochondrial Protein hFis1 Regulates Mitochondrial Fission in Mammalian Cells through an Interaction with the Dynamin-Like Protein DLP1. Mol Cell Biol. 23 (15), 5409-5420 (2003).
- Osellame, L. D., Singh, A. P., et al. Cooperative and independent roles of Drp1 adaptors Mff and MiD49/51 in mitochondrial fission. J Cell Sci. 129 (11), 2170-2181 (2016).
- Esposito, E. A., Shrout, A. L., Weis, R. M. Template-Directed Self-Assembly Enhances RTK Catalytic Domain Function. J Biomol Screen. 13 (8), 810-816 (2008).
- Shrout, A. L., Esposito, E. A., Weis, R. M. Template-directed Assembly of Signaling Proteins: A Novel Drug Screening and Research Tool. Chem Biol Drug Des. 71 (3), 278-281 (2008).
- Celia, H., Wilson-Kubalek, E., Milligan, R. A., Teyton, L. Structure and function of a membrane-bound murine MHC class I molecule. Proc Natl Acad Sci U.S.A. 96 (10), 5634-5639 (1999).
- Li, E., Wimley, W. C., Hristova, K. Transmembrane helix dimerization: Beyond the search for sequence motifs. Biochim Biophys Acta (BBA) – Biomembranes. 1818 (2), 183-193 (2012).
- Zhang, F., Crise, B., Su, B., Hou, Y., Rose, J. K., Bothwell, A., Jacobson, K. Lateral diffusion of membrane-spanning and glycosylphosphatidylinositol- linked proteins: toward establishing rules governing the lateral mobility of membrane proteins. J Cell Biol. 115 (1), 75-84 (1991).
- Ramadurai, S., Holt, A., Krasnikov, V., van den Bogaart, G., Killian, J. A., Poolman, B. Lateral diffusion of membrane proteins. J Am Chem Soc. 131 (35), 12650-12656 (2009).
- Gambin, Y., Reffay, M., et al. Variation of the lateral mobility of transmembrane peptides with hydrophobic mismatch. J Phys Chem. B. 114 (10), 3559-3566 (2010).
- Wilson-Kubalek, E. M., Brown, R. E., Celia, H., Milligan, R. A. Lipid nanotubes as substrates for helical crystallization of macromolecules. Proc Natl Acad Sci. 95 (14), 8040-8045 (1998).

