Rapid Deletion Production in Fungi via Agrobacterium Mediated Transformation of OSCAR Deletion Constructs
Summary
Gene deletion mutants generated through homologous recombination are the gold standard for gene function studies. The OSCAR (One Step Construction of Agrobacterium-Recombination-ready-plasmids) method for rapid generation of deletion constructs is described. Agrobacterium mediated fungal transformation follows. Finally, a PCR based confirmation method of gene deletions in fungal transformants is presented.
Abstract
Precise deletion of gene(s) of interest, while leaving the rest of the genome unchanged, provides the ideal product to determine that particular gene's function in the living organism. In this protocol the OSCAR method of precise and rapid deletion plasmid construction is described. OSCAR relies on the cloning system in which a single recombinase reaction is carried out containing the purified PCR-amplified 5' and 3' flanks of the gene of interest and two plasmids, pA-Hyg OSCAR (the marker vector) and pOSCAR (the assembly vector). Confirmation of the correctly assembled deletion vector is carried out by restriction digestion mapping followed by sequencing. Agrobacterium tumefaciens is then used to mediate introduction of the deletion construct into fungal spores (referred to as ATMT). Finally, a PCR assay is described to determine if the deletion construct integrated by homologous or non-homologous recombination, indicating gene deletion or ectopic integration, respectively. This approach has been successfully used for deletion of numerous genes in Verticillium dahliae and in Fusarium verticillioides among other species.
Introduction
Genetic dissection is a powerful methodology for determining the functional importance of individual or combinations of genes. A standard approach to understand the role of specific genes is production of single gene mutants unaltered in any other gene. The most powerful and least potentially confounding approach is complete and precise deletion of a gene of interest's open reading frame (GOI ORF) without damage to any other gene function.
Because standard ligation approaches for deletion plasmid generation require multiple steps, the rational for OSCAR1 was to produce a more rapid in vitro approach. Figure 1 depicts the assembly process in the OSCAR approach. The method described here has the advantage of combining rapid construction of individual gene deletion vectors in a single multipart reaction in combination with subsequent Agrobacterium tumefaciens mediated transformation (ATMT). OSCAR is very rapid and compares well with other strategies such as use of Gibson assembly in yeast2. The OSCAR method has been used successfully with several Ascomycota species of fungi. These species include: Fusarium verticillioides (unpublished), Verticillium dahliae3, Setosphaeria turcica4, Metarhizium robertsii5, Fusarium oxysporum f. sp. vasinfectum6, Pestalotiopsis microspora7, Colletotrichum higginsianum8, and Dothistroma septosporum9 and Sarocladium zeae (unpublished).
This protocol provides step-by-step instruction for the method including primer design, flank PCR amplification, the OSCAR BP reaction, deletion construct structure confirmation, transformation of Agrobacterium with the construct followed by ATMT based transfer of the deletion construct into the fungal cells, and finally differentiating fungal deletion mutants from those with ectopically integrated deletion constructs.
Protocol
1. Primer Design for PCR Amplification of Gene Flanks
- Download to a word processing file the genomic region of the gene of interest (GOI) including the open reading frame (ORF) and at least 2 kb flanking the gene on each side from FungiDB or other genomic data resource.
- Highlight the ORF intended for deletion and label start and stop codons.
- Identify and highlight adjacent ORFs within the downloaded sequence.
- Use the 2 kb 5' end of the GOI ORF and the primer design tool (see Materials List) to design PCR primer pair O1 and O2 to generate a minimum size product of 1 kb. Take care to not impact adjacent ORFs.
- Paste the 2 kb 5' flank into the "Sequence Entry" window. Click on "Show Custom Parameters" box. Enter the following custom design parameters: primer TM 58 (min), 60 (opt) and 62 (max); Primer GC 40 (min), 50 (opt) and 60 (max); Primer Size 22 (min), 24 (opt) and 26 (max); Amplicon Size 1250 (min), 1500 (opt) and 2000 (max).
- Choose the result that places the reverse primer (O2) closest to the ORF. Capture a screen shot of the assays and copy and paste the primer sequences into the word processing document.
- Repeat the process for 3' flank to generate primers O3 and O4, this time choose the result placing the forward primer (O3) closest to the target ORF.
- Add appropriate attach sites to 5' ends of primers 1 through 4 (see Table 1).
- Also design and order ORF specific primers to be used for deletion confirmation in section 4 below. Parameters should be the same as in step 1.4.1 except use Amplicon Size 500 (min), 750 (opt) and 1000 (max) adjusting for length of ORF if necessary. Finally, for confirmation of homologous recombinants, generate 5' out and 3' out primers found just outside the flanks within the chromosome. These "out" primers will be used with hygR (210) and hygF (850), respectively (Fig 2).
- Order primers.
2. Production of OSCAR Constructs
- Using a hi-fidelity Taq, carry out two reactions, one each for the 5' and 3' flanks, using primers (100 µM) O1 and O2 in one reaction and primers O3 and O4 in the second. Reaction mixture contains the following: 29.5 µL sterile distilled water (SDW), 5 µL 10x LA PCR buffer, 8 µL 2.5 mM dNTP mix, 5 µL 25 mM MgCl2, 1 µL template (50 ng/µL), 0.5 µL hi-fidelity Taq, 0.5 µL Primer O1 and 0.5 µL Primer O2 for 5' flank (or 0.5 µL Primer O3 and 0.5 µL Primer O4 for 3' flank) Use reaction conditions are as follows: 94 °C for 1 min, then 30 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 2 min, then final steps of 72 °C for 5 min and then hold indefinitely at 10 °C.
- Run 0.8% agarose 1x TAE (40 mM Tris acetate pH 8.3, 1 mM EDTA) gel electrophoresis for approximately 125 Vh in a standard minigel apparatus to determine product size and relative concentration. Post stain the gel with non-ethidium stain according to the manufacturer instructions. Visualize on a UV illuminator.
- If 5' and 3' flank products are in roughly even concentration, combine them and use an affinity column kit (or PEG precipitation 90 µL combined PCR products, 240 µL TE buffer pH 7.5, 160 µL of 30% polyethylene glycol PEG8000 30 mM MgCl2) to co-purify the PCR products according to the manufacturer's protocol. If they are not of equal concentration combine all of low concentration product with an estimated equal amount of product from the higher yield reaction.
- Use a spectrophotometer to measure purified DNA concentration.
- Carry out BP reaction by mixing the following: 1 µL of 60 ng/µL pOSCAR, 2 µL of 60 ng/µL pA-Hyg-OSCAR, 1 µL of 60 ng/µL combined flanks, 1 µL of clonase. Incubate for 16 h at 25 °C. Terminate the reaction by adding 0.5 µL proteinase K and incubate for 10 min at 37 °C.
- Transform high competence E. coli cells. Both commercially available competent cells and home-made DH5α CaCl2 competent cells were used successfully as recipients as described by the manufacturer's instructions. Plate on low sodium (0.5 g/L NaCl) LB containing 100 µg/mL spectinomycin (Spec).
- Identify the correct construct by performing DNA minipreps10 of colonies generated above. Perform double digestion with HindIII and KpnI as follows: pipette 5 µL DNA, 2 U of each enzyme, 2 µL appropriate 10x buffer with SDW to a 20 µL total volume. This releases the insert (approximately 4.5 kb with 1.5 kb gene flanks) from the vector (ca. 7 kb) and cuts any sites in flanks which can give predictable band sizes.
- Sequence11 select clones showing an appropriate banding pattern.
3. Agrobacterium tumefaciens Mediated Transformation (ATMT) of Fungi
- Transform Agrobacterium tumefaciens strain AGL1 with OSCAR deletion construct12.
- Transform fungus with AGL1 containing the GOI OSCAR deletion plasmid. To do this carry out the following steps:
- Prepare a fungal spore suspension with sterile water at 2 x 106 spores/mL. Quantify spores on a hemocytometer or automated cell counter. Place 500 µL of this in a 1.5 mL microcentrifuge tube. This set up is for 4 transformation plates.
- Use a blue sterile disposable loop to add an easily visible gob (should cover about 20% of the loop diameter) of the deletion plasmid containing AGL1 from 2-3 day old LB-Spec 100 medium (see Materials List for composition) containing plates and mix into the spore suspension. Vortex till bacterial cells are well dispersed (ca. 5 min medium speed).
- Pipette 100 µL of the conidia-Agro suspension onto the center of a cellulose membrane filter placed on each of four 6 cm Petri dishes containing co-cultivation medium12.
- Spread the suspension with sterile glass beads (about 4) to cover the whole surface of the membrane filter. Alternatively spread the suspension using a traditional spreader tool. Allow the membrane filter to dry in a hood for approximately 10 min, wrap with paraffin film. Repeat steps 3.2.2 and 3.2.3 to set up multiple transformations.
- Incubate the plate for 2 days at room temperature, inverted.
- Transfer the membrane filter onto 6 cm Aspergillus selection medium12 plates containing hygromycin B (Hyg) 150 µg/mL, 200 mM cefotaxime and 100 µg/mL moxalactam. Incubate at room temperature for 5-7 days before isolating Hyg resistant colonies.
- With sterile toothpicks, transfer putative transformants onto 6 cm Potato Dextrose Agar (PDA) plates containing 150 µg/mL Hyg, 100 µg/mL kanamycin.
4. Deletion Mutant Identification by PCR
- Extract DNA for PCR from transformants by thermolysis13.
- Once transformants form colonies on the PDA from step 3.2.7, use a sterile toothpick to transfer a small amount (2 mm x 2 mm) of mycelium or yeast cells from the colony into 100 µL of lysis solution (50 mM sodium phosphate, pH 7.4, 1 mM EDTA, 5% glycerol) in a microcentrifuge tube.
- Incubate the mixture at 85-90 °C for 20-30 min. Store the crude extract containing genomic DNA at -20 °C until use in step 4.2.
- Carry out 4 PCR reactions per transformant, one each to detect homologous recombination of the 5' and 3' flanks, respectively, one for the selectable marker (hygromycin phosphotransferase in this case) and one for the GOI ORF.
NOTE: We generally use inexpensive low-fidelity Taq for these reactions. Reaction conditions are as described in step 2.1 above and contain SDW 20 µL, 10x Taq buffer 2.5 µL, dNTPS (10 mM) 0.5 µL, homemade Taq14, 0.5 µL, Primer A (100 µM) 0.5 µL, Primer B (100 µM) 0.5 µL, Template 0.5 µL. Lower concentration primer stocks (10 µM) can be used equally well. - Run an agarose gel (e.g. 0.8%) of the PCR products. Deletion mutants will produce the Hyg band but not an ORF product. Ectopic integrants will show both bands. Wild type will produce only the ORF band.
- Permanently store deletion mutants (and an ectopic transformant or two for controls) for future use.
NOTE: 15% glycerol is used to store our strains long-term at -80 °C.
Representative Results
The OSCAR method, in a single reaction, generates a plasmid containing the flanks of the target gene to be deleted surrounding the selectable marker cassette. The production of deletion constructs using OSCAR is very efficient. The system can, however, produce partial constructs containing some but not all three fragments (the two gene flanks and the selectable marker). Generally, the majority of E. coli transformants contain the correct OSCAR construct. For example, Figure 2 depicts the OSCAR deletion construct for the Verticillium dahliae gene VDAG_06812. In this case the flanks of VDAG_06812 lack HindIII or KpnI sites and therefore a plasmid insert of 4,247 bp should be released. Figure 3 shows HindIII-KpnI restriction enzyme double digestion confirmation of the correct plasmid structure except for lanes 5 and 11 representing anomalous constructs. Figure 4 shows final confirmation of two different gene deletions by Southern blot. Figure 5 shows a definitive PCR approach as an alternative to Southern blot.
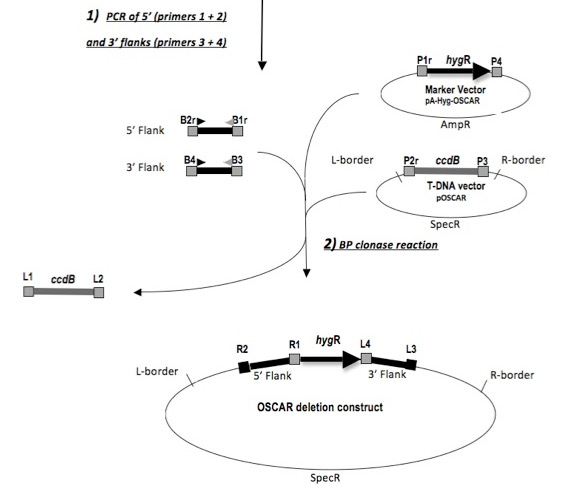
Figure 1: OSCAR deletion construction reaction. The OSCAR deletion construction process includes separate PCR amplification of the 5' and 3' gene flanks, followed by a BP clonase reaction with the combined purified flank products and the binary and selection marker plasmids. B1r, B2r, B3, B4, P1r, P2r, P3, P4, R1, R2, L3 and L4 represent lambda recombination sites. Re-print with permission from reference1.
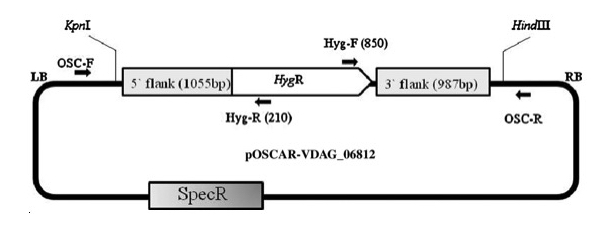
Figure 2: OSCAR deletion construct for Verticillium dahliae gene VDAG_06812. Positions of restriction enzyme recognition and primer annealing sites to verify correct deletion construct structure are shown. Re-print with permission from reference1.
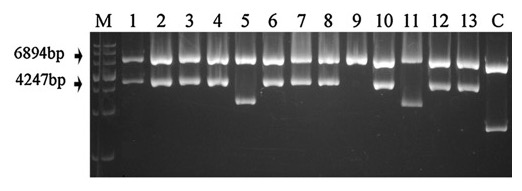
Figure 3: Restriction digestion confirmation of the deletion construct structure of VDAG_06812 with KpnI and HindIII. Plasmids were double digested and analyzed by gel electrophoresis on a 0.8% agarose gel. The correct construct generates two bands, of about 6.9 kb and 4.2 kb, representing the binary vector backbone and the HygR marker fused to the target gene flanks, respectively. Re-print with permission from reference1.
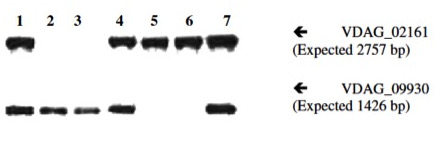
Figure 4: Southern blot hybridization confirming deletion of VDAG_02161 and VDAG_09930 in V. dahliae using OSCAR deletion constructs. Genomic DNAs were digested with BclI. They were then probed simultaneously with VDAG_02161 and VDAG_09930 ORF probes. Hybridization bands are 2,757 bp and 1,426 bp for the wild type alleles of VDAG_02161 and VDAG_09930, respectively. Samples are as follows: lane 1: wild type strain VdLs.17; lane 2: deletion mutant strain 02161.1; lane 3: deletion mutant strain 02161.3; lane 4: ectopic transformant strain Ect 02161.2; lane 5: deletion mutant strain 09930.3; lane 6: deletion mutant strain 09930.10; lane 7: ectopic transformant strain Ect 09930.4. Re-print with permission from reference1.
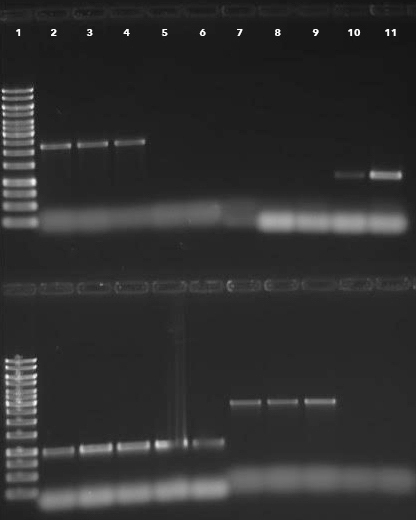
Figure 5: Deletion mutant confirmation by PCR. Analysis of deletion and ectopic Fusarium verticillioides transformants for gene FVEG_13253. Lanes 1 top and bottom, marker; lanes 2-6 top gel transformant PCR 5' out fragment amplification; lanes 7-11 top gel ORF amplification; lanes 2-6 bottom gel Hyg gene amplification; lanes 7-11 bottom gel 3' out fragment amplification. Samples are deletion strains 11/31 (lanes 2 and 7), 11/32 (lanes 3 and 8), 11/33 (lanes 4 and 9), and ectopic transformants 11/34 (lanes 5 and 10), and 11/35 (lanes 6 and 11).
| Primer | Use | Sequence |
| Primer O1-(attB2r) | Amplification of 5´ flank, primer forward | 5’-GGGGACAGCTTTCTTGTACAAAGTGGAA |
| Primer O2-(attB1r) | Amplification of 5´ flank, primer reverse | 5’-GGGGACTGCTTTTTTGTACAAACTTGT |
| Primer O3-(attB4) | Amplification of 3´ flank, primer forward | 5’-GGGGACAACTTTGTATAGAAAAGTTGTT |
| Primer O4-(attB3) | Amplification of 3´ flank, primer reverse | 5’-GGGGACAACTTTGTATAATAAAGTTGT |
Table 1: Specific OSCAR primer 5' extensions added to gene specific primer sequences.
| Primer | Use | Sequence |
| OSC-F | OSCAR forward sequencing primer, sequences sense strand of 5’ flank | 5’-CTAGAGGCGCGCCGATATCCT |
| OSC-R | OSCAR reverse sequencing primer, sequences anti-sense strand of 5’ flank | 5’-CGCCAATATATCCTGTCAAACACT |
| HygR (210) | Reverse sequencing primer, sequences anti-sense strand of 5’ flank | 5’-GCCGATGCAAAGTGCCGATAAACA |
| HygF (850) | Forward sequencing primer, sequences sense strand of 3’ flank | 5’-AGAGCTTGGTTGACGGCAATTTCG |
| New Hyg Marker Forward | To amplify the hygromycin resistance gene as a control (together with New Hyg Marker Reverse primer below) | 5'-GACAGGAACGAGGACATTATTA |
| New Hyg Marker Reverse | To amplify the hygromycin resistance gene as a control (together with New Hyg Marker Forward primer above) | 5'-GCTCTGATAGAGTTGGTCAAG |
Table 2: Primers for analysis of OSCAR deletion constructs.
Discussion
One Step Construction of Agrobacterium-Recombination-ready-plasmids (OSCAR) has been successfully employed with an ever-increasing number of Ascomycota fungi. The method should also easily be applicable to the Basidiomycota and species from other fungal phyla (with appropriate promoters driving selectable marker genes), assuming Agrobacterium mediated transformation and homologous recombination are possible. Additional marker vectors have been generated to diversify choices of anti-fungal compound as well as allow the production of double and higher order mutants. These include resistance to G418, nourseothricin, and recently the herbicides glufosinate ammonium and chlorimuron ethyl4.
The method was worked out for use with Verticillium dahliae from which the images presented here are primarily derived1. More recently OSCAR has been used extensively for deletion of genes in the mycotoxigenic fungus Fusarium verticillioides. Over 60 deletion constructs have been made and deletions mutants for more than 30 genes generated (unpublished).
In its current iteration the process requires about 4-6 weeks to have a set of confirmed deletion mutants in hand. The following is a brief list of the major OSCAR steps and approximate time needed to accomplish them: 1) Design and procurement of OSCAR primers based on genome data (5 days), 2) flank amplification and cloning, (2 days), 3) clone confirmation by miniprep and sequencing (1 week), 4) introduction of plasmid into Agrobacterium for ATMT (4 days), 5) production of transformants by ATMT in Fusarium verticillioides and grow out (2 weeks, note that this is dependent on growth rate of the fungus under study), thermolysis and PCR determination of transformant genotypes (2 days), 6) single sporing, genotype confirmation and storage (1-2 weeks).
The adaptation of OSCAR to true high throughput has not been attempted to our knowledge but should be practical. The reactions are sufficiently robust to allow for successful application in multi-well format. The use of robotics would certainly advance this approach. The OSCAR method has the time saving advantage of requiring a single recombination reaction containing the amplified flanks and two plasmids pOSCAR and pA-Hyg OSCAR. Similar approaches require generation of an entry clone that has to be confirmed and then used in a second reaction to generate the construct of interest. Plasmids pOSCAR and pA-Hyg OSCAR mentioned are available via Addgene or the Fungal Genetics Stock Center15. Other OSCAR marker plasmids such as pA-Bar-OSCAR and pA-Sur-OSCAR may be available from authors5.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank the following undergraduate and high school students for their work to generate OSCAR mutants in Fusarium verticillioiodes: Anjellica Miller, Athar Naseer, Xiu Lin, Katelyn Woodburry, Chelsea Patterson, Kathleen Robertson, Krystina Bradley, Ashton Rogers, Alexis McKensie, Manny Hernandez, Ashli Crepsac, Jeff Delong, Christian King, Gi Jeong, Maria Belding, Christy Burre, Daniel O'Meara, Lauren (Victoria) Cook, Jake Goodman, Sampriti De, Oge Okoye, Alyssa Beckstead, Garrett Hibbs, Nick Goldstein, Caroline Twum, Chris Benson, Louis Stokes, Hannah Itell, Jane Hulse, Jasim Mohammed, James Loggins, Kelli Russell, Gre'Nisha Jones, Kristin Sheaffer, Mariam Hammady, Ava Wilson, Katrina Bazemore, Toney Harper, Karlin McGhee, Mohmed Momin, Rima Momin, Thi Ngoc Le and Angel Pham.
Materials
| FungiDB | Database/ http://fungidb.org/fungidb/ | ||
| IDT PrimerQuest | IDT | Primer design online software/ http://www.idtdna.com/Primerquest/Home/Index | |
| Microsoft Word | Sequence file manipulation | ||
| Low Na LB Spec 100 medium | E. coli transformant selection, composition: 1% tryptone, 0.05% NaCl, 0.5% yeast extract, 1.5 % agar if for solid medium | ||
| Co-cultivation medium | ATMT transformation induction (Reference 12) | ||
| Aspergillus minimal medium with Hygromycin | Fungal transformant selection | ||
| PDA medium | Acumedia | 7149A | Single spore slant tubes |
| PDA-Hyg-Kan medium | Fungal ransformant isolation, PDA containing 150 μg/ml hygromycin B and 100 μg/ml Kanamycin; | ||
| Glass beads | Genlantis | C400100 | Plate spreading |
| Nitrocellulose filters (47mm) | Fisher | 09-719-555 | Co-culturing for ATMT |
| Various centifuge tubes | multiple preps | ||
| Petri plates (various) | Culturing of bacteria and Fungi | ||
| pA-Hyg OSCAR | Addgene | 29640 | Selectable marker vector |
| pOSCAR | Addgene | 29639 | Assembly vector |
| DH5a One Shot Competent E. coli cells | Life Technologies | 12297-016 | BP reaction transformation |
| ccdB survival E. coli cells | Life Technologies | A10460 | Maintenance of pOSCAR |
| Wooden transfer sticks | Colony streaking | ||
| Toothpicks | Colony picking | ||
| Microcentrifuge | Pelleting Bacteria etc | ||
| Preparative centrifuge | Fungal spore collection | ||
| Dissecting microscope | Single spore isolation | ||
| Automated Cell Counter | Spore suspension calculation | ||
| Compound microscope | Hemocytometer cell counting | ||
| QIAquick PCR Purification Kit | Qiagen | 28104 | PCR gene flank produict purification |
| TaKaRa LA Taq | Takara Bio USA | RR002A | Hi Fidelity taq polymerase for OSCAR flank generation |
| Hygromycin B | InvivoGen | ant-hg-5 | |
| Spectinomycin | Sigma | 22189-32-8 | |
| Cefotaxim | TCI America | C2224 | |
| Moxalactam | Sigma-Aldrich | 43963 | |
| GelRed | Phenix Research Products | RGB-4103 | Post staining agarose gels |
| Qiagen QIAquick PCR Purification Kit (Cat. No. 28104) | |||
| (OneShot_ Mach1TM T1R or One Shot_ OmniMAX™ 2 T1R from Invitrogen) | Thermo Fisher Scientific | C862003 | |
| Gateway BP Clonase II Enzyme mix | Thermo Fisher Scientific | 11789020 | Used to assemble deletion construct in pOSAR |
| PrimerQuest tool | IDT | Used in step 1.4; available on http://www.idtdna.com/Primerquest/Home/Index |
References
- Paz, Z., García-Pedrajas, M. D., Andrews, D. L., Klosterman, S. J., Baeza-Montañez, L., Gold, S. E. One step construction of Agrobacterium-Recombination-ready-plasmids (OSCAR), an efficient and robust tool for ATMT based gene deletion construction in fungi. Fungal Genet Biol. 48 (7), 677-684 (2011).
- Gibson, D. G., Young, L., Chuang, R. Y., Venter, J. C., Hutchison, C. A., Smith, H. O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 6 (5), 343-345 (2009).
- Klosterman, S. J., et al. Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog. 7 (7), (2011).
- Xue, C., Wu, D., Condon, B. J., Bi, Q., Wang, W., Turgeon, B. G. Efficient gene knockout in the maize pathogen Setosphaeria turcica using Agrobacterium tumefaciens-mediated transformation. Phytopathology. 103 (6), 641-647 (2013).
- Xu, C., et al. A high-throughput gene disruption methodology for the entomopathogenic fungus Metarhizium robertsii. PloS One. 9 (9), (2014).
- Crutcher, F. K., Liu, J., Puckhaber, L. S., Stipanovic, R. D., Bell, A. A., Nichols, R. L. FUBT, a putative MFS transporter, promotes secretion of fusaric acid in the cotton pathogen Fusarium oxysporum f. sp. vasinfectum. Microbiology. 161, 875-883 (2015).
- Yu, X., Wang, Y., Pan, J., Wei, D., Zhu, X. High frequency of homologous gene disruption by single-stranded DNA in the taxol-producing fungus Pestalotiopsis microspora. Ann Microbiol. 65 (4), 2151-2160 (2015).
- Korn, M., Schmidpeter, J., Dahl, M., Müller, S., Voll, L. M., Koch, C. A Genetic Screen for Pathogenicity Genes in the Hemibiotrophic Fungus Colletotrichum higginsianum Identifies the Plasma Membrane Proton Pump Pma2 Required for Host Penetration. PloS One. 10 (5), e0125960 (2015).
- Chettri, P. . Regulation of dothistromin toxin biosynthesis by the pine needle pathogen Dothistroma septosporum: a thesis presented in the partial fulfilment of the requirements for the degree of Doctor of Philosophy (PhD) in Genetics at Massey University, Manawatu, New Zealand . , (2014).
- Chen Zhou, ., Yujun Yang, ., Jong, A. Y. Mini-prep in ten minutes. Biotechniques. 8 (2), 172 (1990).
- Sanger, F., Nicklen, S., Coulson, A. R. DNA sequencing with chain-terminating inhibitors. P Natl Acad SciUSA. 74 (12), 5463-5467 (1977).
- Khang, C. H., Park, S. Y., Rho, H. S., Lee, Y. H., Kang, S., Wang, K. a. n. Filamentous fungi (Magnaporthe grisea and Fusarium oxysporum). Agrobacterium Protocols. 2, 403-420 (2007).
- Zhang, Y. J., Zhang, S., Liu, X. Z., Wang Wen, H. A., M, A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains. Lett Appl Microbiol. 51 (1), 114-118 (2010).
- Pluthero, F. G. Rapid purification of high-activity Taq DNA polymerase. Nucleic Acids Res. 21 (20), 4850-4851 (1993).
- McCluskey, K. Boosting Research and Industry by Providing Extensive Resources for Fungal Research. Gene Expression Systems in Fungi: Advancements and Applications. , 361-384 (2016).

