Recording EEG in Freely Moving Neonatal Rats Using a Novel Method
Summary
Here, we introduce a novel technique designed to record electroencephalography (EEG) in freely moving neonatal epileptic pups and describe its procedures, features, and applications. This method allows one to record EEG for more than 1 week.
Abstract
EEG is a useful method to detect electrical activity in the brain. Moreover, it is a widely used diagnostic tool for various neurological conditions, such as epilepsy and neurodegenerative disorders. However, it is technically difficult to obtain EEG recordings in neonates as it requires specialized handling and great care. Here, we present a novel method to record EEG in neonatal rat pups (P8-P15). We designed a simple and reliable electrode using computer pin loci; it can be easily implanted into the skull of a rat pup to record high-quality EEG signals in the normal and epileptic brain. Pups were given an intraperitoneal (i.p.) injection of the neurotoxin kainic acid (KA) to induce epileptic seizures. The surgical implantation performed in this procedure is less expensive than other EEG procedures for neonates. This method allows one to record high-quality and stable EEG signals for more than 1 week. Furthermore, this procedure can also be applied to adult rats and mice to study epilepsy or other neurological disorders.
Introduction
It is well established that continuous communication between neurons is required to obtain normal brain function. The interneuronal communication primarily takes place at synapses, where information from one neuron is conveyed to a second neuron. This synaptic transmission is mediated by two types of dedicated structural arrangements: electrical or chemical synapses 1. Electrophysiology is the field that captures the electrical potential produced during interneuronal communication that control overall body functions and behavior 2. EEG is the most commonly used method among many electrophysiological techniques.
EEG is a technique used to detect changes in electrical signals produced by internal or external stimuli. Moreover, it is an essential test for clinical diagnosis and outcome prediction of various neurological conditions such as epilepsy, Parkinson's and Alzheimer's disease, as well as effects of pharmacological and toxicological agents 3. Generally, an epileptic patient shows hyperexcitability and impaired functional connectivity within the brain; these are summarized as interictal epileptiform discharges (IEDs) and can be recorded by EEG in the form of sharp, transient spikes; sharp waves; spike-wave complexes; or polyspikes 4. The main feature of the epileptic brain is the spontaneous occurrence of epileptic seizures, which can be recorded either from the scalp or from the brain parenchyma in order to locate the brain area responsible for the seizures 5. Furthermore, EEG also has very important implications in neurodegenerative disorders like Alzheimer's disease (AD). Research suggests that altered EEG recordings and impaired oscillatory networks in AD patients are common. However, our knowledge about the pathophysiology of network oscillations in neurodegenerative diseases is surprisingly incomplete and needs to be further explored 6.
In this protocol, we have designed a simple electrode with which one can record EEG to understand the electrical communication in both the normal and the pathological brain. The surgical implantation in this method is cheaper than other available procedures 7. Moreover, this method can be used to record high-quality and stable EEG signals for longer timeframes (i.e., 2-4 h every day for 1 week). In addition, we used lighter electrodes (weighing approximately 26 mg) that enable the animals to behave more naturally 8. This method is widely applicable to the study of EEG in neonatal rat pups that requires the amplifier and digitizer, commonly used in electrophysiology lab and does not require any additional devices.
Protocol
Animal care, surgical procedure, and recording procedures were in accordance with the guidelines for the South China Normal University Animal Care and Use Committee.
1. Electrode Preparation (Figure 1A-C)
NOTE: Computer pin loci is simply a pronged contact as a part of signal interface in communication devices. It consists of a male connector that plugs into the female connector.
- Carefully separate the male and female pins from the computer pin loci (Figure 1A) with the help of a pincer. Connect the male and female pins together to form an electrode and apply cyanoacrylate to create a strong adhesive bond (Figure 1C).
- Put the electrodes into a beaker filled with distilled water for and place it to ultrasonic cleaner for 10 min. Move them to a drying oven at 45 °C for 30 min. Sterilize the electrodes using UV light for 30 min.
2. Surgical procedure (Figure 1D-F)
- Prepare the sterilized surgical instruments and stereotaxic apparatus. Anesthetize the neonatal rat pup using isoflurane anesthesia (2.5 %) with air. When the pup is deeply anesthetized, adjust the dose of isoflurane to 1.0%. Perform a tail or toe pinch prior to the surgery to ensure the proper depth of anesthesia.
- Fix the head of the pup in the stereotaxic apparatus by placing the ear bars into the ear canals and slightly tightening them.
NOTE: Do not excessively tighten the ear bars, as the neonatal skull is very soft. - Maintain the sterile surgical field by spraying all equipment with 70 % ethanol. Make a 15-mm incision on the head using a scalpel. Using forceps, gently pull the scalp away from the midline at the four corners. Put some saline-soaked cotton below the skin to keep the incision wide open (Figure 1D).
- Find the bregma and lambda points on the skull and mark them with a pencil. Use a syringe needle (26 G) to make two burr holes into the prefrontal cortex (PFC) and hippocampus.
NOTE: The PFC is located at +1.8 mm posterior to bregma and -0.5 mm lateral to the midline, while the hippocampus is located at -2.0 mm anterior to bregma and ±0.5 mm lateral to the midline (Figure 1D and E). The depth of the electrode should not be more than 2 mm below the cortical surface to minimize brain damage. - Use forceps to hold the electrodes and insert the reference and recording electrodes into the PFC and hippocampus, respectively. Apply erythromycin ointment around the electrode to avoid any possible infection. Fix the electrode using cyanoacrylate.
- Prepare dental acrylic cement so that it has a gluey and viscous consistency. Apply the dental cement to cover the electrodes and the rest of the skull.
NOTE: Thoroughly dry the skull before applying the dental cement. - Apply 5% picric acid onto the electrodes to protect them.
NOTE: The entire procedure should be done in a biosafety hood to maintain sterile conditions. - Remove the animal from the stereotaxic frame and inject 300 µL of 10 % glucose subcutaneously. Place it on a heated blanket for recovery. Make sure that the animal is warm (37 °C) and ambulatory (i.e., completely recovered). Administer buprenorphine intraperitoneally (0.05 mg/kg) for post-surgical pain.
NOTE: Do not leave an animal unattended until it has regained sufficient consciousness (i.e. normal behavior and movement). - Return the pup to its home cage with their dam after it regains consciousness. Wait for two days until the animal is fully recovered.
3. EEG Recording
- After full recovery, connect the electrodes implanted on the skull of the pup to the amplifier in its own cage. Connect the amplifier to an analogue-to-digital converter and attach the converter to a computer; the connecting lines should be treated carefully so that they do not get tangled.
- Select at least 10,000 Hz sampling rate on the data acquisition unit for recording (the bandwidth of the transmitter is 1-100 Hz). Ensure that the data is sampled properly.
- After getting the baseline recording, inject the pup intraperitoneally with kainic acid (KA) (2 mg/kg) to induce epileptic seizures. 15 min after the KA injection observe and record the epileptic discharges. Seizure produce through KA are usually physical.
NOTE: The ictal-tonic duration is about 15.2 ±0.9 s, seizure duration is about 62±5 s. Seizures can be prevented in the neonatal rat by giving IP injection of chloral hydrate (400 mg/kg). - Save the digitized data and analyze it using signal-processing software packages, such as spike2. Reveal the power level of different frequency components in the neonatal EEG signal by performing a power spectrum analysis. Calculate the power in a 1-min timeframe by finding the root-mean-square amplitude from 1 to 100 Hz (EEG band) 9.
Representative Results
If the above surgical procedures are conducted properly, one channel rat neonatal pup EEG recording will be successfully performed. 10 min after the KA injection, a regular pattern of behavioral signs emerged in the form of irregular movements and scratching, tremors, and loss of balance. Figure 2 shows the representative raw EEG traces and interictal, ictal-tonic, and ictal-clonic expanded traces. Recurrent interictal and ictal EEG discharge patterns started 15-60 min (30 ±5.2 min; mean ±SEM) after KA injection (Figure 2A and 2B). Figure 2D and 2E represents the tonic-clonic seizures that are the most common type. The ictal-tonic duration lasted 15.2 ±0.9 s (Figure 2D). The representative results showed that tonic discharges were followed by clonic bursts (Figure 2B, 2D, and 2E).
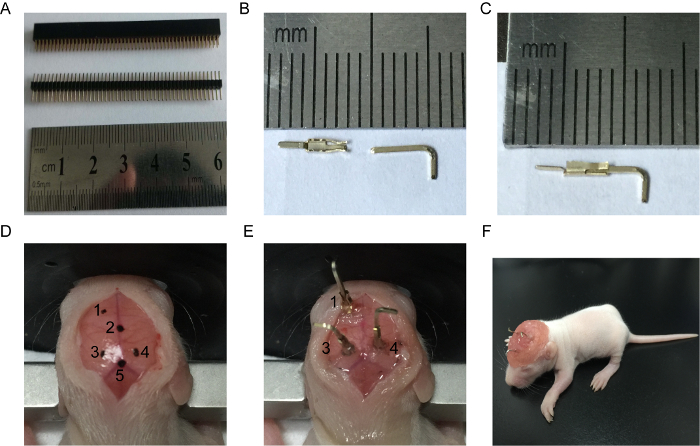
Figure 1: Illustration of Surgical Implantation Protocol. (A) Tiny computer pins. (B) Female pin (left) and male pin (right). The scale bar is used to measure the length of the female pin. (C) Connected female and male pin. (D) Exposed skull. No. 1 presents the reference site, No. 2 presents Bregma, No. 3 and 4 present recording sites, and No. 5 presents Lambda. (E) Electrode implantation. No. 1 presents the reference electrode, No. 3 and 4 present the recording electrode. (F) Recovered rat pup (P9). Please click here to view a larger version of this figure.
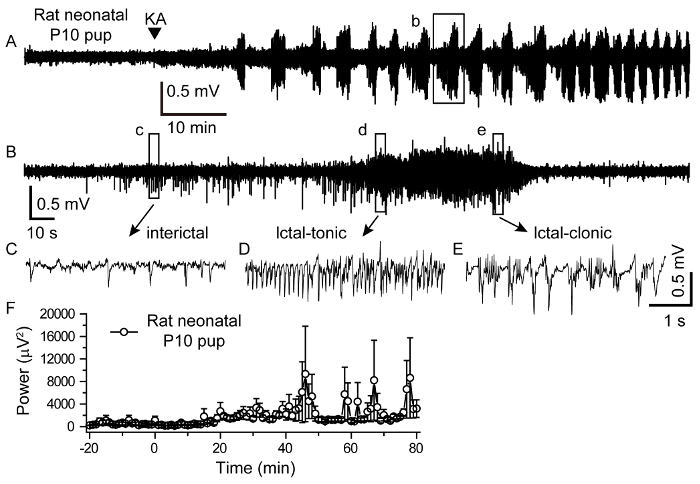
Figure 2: KA-induced Neonatal Seizure. (A) A representative EEG trace from a P10 rat that was injected with KA (2 mg/kg, i.p.) showed recurrent epileptic burst discharges. (B) Extended view of single cluster-like epileptic discharges from the box in A. (C, D and E) Typical interictal, ictal-tonic, and ictal-clonic traces expanded from the box in B. (F) Summarized data showing the average EEG power (1 min time window) before and after the KA injection (mean ±SEM). Please click here to view a larger version of this figure.

Figure 3: EEG Recording Set-up. Representative trace recording is shown in freely moving neonatal rat pup. Please click here to view a larger version of this figure.
Discussion
Here, we report surgical and recording procedures to acquire EEG in freely moving neonatal rat pups by wired method (Figure 3). It has been suggested that the P7-P12 rat pup is at the developmental age that corresponds to a full-term human neonate 10,11. It is technically difficult to obtain high-quality EEG recording data when working with rat pups in this age group. In addition, it requires specialized handling and great care 8.
Previous studies examining in vivo EEG recordings in neonatal rats have been conducted using wired recording solutions in P12 or older animals 12, but these approaches are very costly. By relying on simple electrodes, we have been able to make an electrode (Figure 1) that reduces the cost of the experiment to as little as $1 while still giving high-density EEG recordings. Surgical implantation in this method can take as little as 10 min, depending on the complexity of the surgery.
The critical steps in this protocol is the implantation of electrodes. It should be done carefully. To maintain constant contact of the electrode with the skull, the following steps are important to consider. Firstly, remove the tissue debris on the skull with the help of sterilized cotton swab because it may set apart the electrode from the skull. Secondly, use cyanoacrylate properly to attach the electrode with the skull. Thirdly, keep the adequate viscosity of dental cement. It is better to apply dental cement in two layers; first layer should be a little dense to cover the skull while the second layer should be a little runny to cover the corners of first layer. Tap the dental cement gently with a pipette tip to settle it down to make the strong adhesive bond. If it is too watery it may build an insulating layer under the electrode, and if it is too dense, the dental cement will easily fall off from the skull due to its own weight. It is also important to maintain the pup's body temperature at 37 °C throughout the surgery for survival.
The application of this procedure is limited to recording EEG in neonatal rat pups between the ages of P8 and P15 because the growing pups may remove the dental cement and electrode from their skull. In terms of further applications, this method can also be used to record the electrical activity of adult rats and mice after minor modification. Moreover, this method lends itself well to use in various behavioral experiments, such as the elevated plus maze.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Natural Science Foundation of China (31171355) and the Natural Science Foundation of Guangdong (S2011010003403, 2014A030313440).
Materials
| Computer pin | |||
| Pincer | DELI Group Co., Ltd. | ||
| 502 super glue | DELI Group Co., Ltd. | 7144 | |
| Drying oven | Boxun | GZX-9140MBE | |
| Isofluorane | RWD Life Science | 902-0000-522 | |
| Stereotaxic apparatus | RWD Life Science | 900-0068-507 | |
| Anesthesia apparatus | RWD Life Science | 902-0000-510 | |
| Homeothermic Heating Device | Harvard Apparatus | K 024509 | |
| Amplifier Model 3000 | A-M Systems | 61558 | |
| Micro1401 Analog Digital converter | Cambridge Electronic Design Ltd. | 4383 | Data acquisition unit |
| Spike2 | Cambridge Electronic Design Ltd. |
References
- Pereda, A. E. Electrical synapses and their functional interactions with chemical synapses. Nat Rev Neurosci. 15 (4), 250-263 (2014).
- Chorev, E., Epsztein, J., Houweling, A. R., Lee, A. K., Brecht, M. Electrophysiological recordings from behaving animals–going beyond spikes. Curr Opin Neurobiol. 19 (5), 513-519 (2009).
- Freeborn, D. L., McDaniel, K. L., Moser, V. C., Herr, D. W. Use of electroencephalography (EEG) to assess CNS changes produced by pesticides with different modes of action: effects of permethrin, deltamethrin, fipronil, imidacloprid, carbaryl, and triadimefon. Toxicol Appl Pharmacol. 282 (2), 184-194 (2015).
- Werhahn, K. J., Hartl, E., Hamann, K., Breimhorst, M., Noachtar, S. Latency of interictal epileptiform discharges in long-term EEG recordings in epilepsy patients. Seizure. 29, 20-25 (2015).
- Staba, R. J., Stead, M., Worrell, G. A. Electrophysiological biomarkers of epilepsy. Neurotherapeutics. 11 (2), 334-346 (2014).
- Nimmrich, V., Draguhn, A., Axmacher, N. Neuronal Network Oscillations in Neurodegenerative Diseases. Neuromolecular Med. 17 (3), 270-284 (2015).
- Zayachkivsky, A., Lehmkuhle, M. J., Dudek, F. E. Long-term Continuous EEG Monitoring in Small Rodent Models of Human Disease Using the Epoch Wireless Transmitter System. J Vis Exp. (101), e52554 (2015).
- Zayachkivsky, A., Lehmkuhle, M. J., Fisher, J. H., Ekstrand, J. J., Dudek, F. E. Recording EEG in immature rats with a novel miniature telemetry system. J Neurophysiol. 109 (3), 900-911 (2013).
- Dzhala, V. I., et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 11 (11), 1205-1213 (2005).
- Tucker, A. M., Aquilina, K., Chakkarapani, E., Hobbs, C. E., Thoresen, M. Development of amplitude-integrated electroencephalography and interburst interval in the rat. Pediatr Res. 65 (1), 62-66 (2009).
- Savard, A., et al. Involvement of neuronal IL-1beta in acquired brain lesions in a rat model of neonatal encephalopathy. J Neuroinflammation. 10, 110 (2013).
- Cuaycong, M., et al. A novel approach to the study of hypoxia-ischemia-induced clinical and subclinical seizures in the neonatal rat. Dev Neurosci. 33 (3-4), 241-250 (2011).

