Quadruple Immunostaining of the Olfactory Bulb for Visualization of Olfactory Sensory Axon Molecular Identity Codes
Summary
Olfactory sensory neurons express a wide variety of axon-sorting molecules to establish proper neural circuitry. This protocol describes an immunohistochemical staining method to visualize combinatorial expressions of axon-sorting molecules at the axon termini of olfactory sensory neurons.
Abstract
The mouse olfactory system is often used to study mechanisms of neural circuit formation because of its simple anatomical structure. An Olfactory Sensory Neuron (OSN) is a bipolar cell with a single dendrite and a single unbranched axon. An OSN expresses only one Olfactory Receptor (OR) gene, OSNs expressing a given type of OR converge their axons to a few sets of invariant glomeruli in the Olfactory Bulb (OB). A remarkable feature of OSN projection is that the expressed ORs play instructive roles in axonal projection. ORs regulate the expression of multiple axon-sorting molecules and generate the combinatorial molecular code of axon-sorting molecules at the OSN axon termini. Thus, to understand the molecular mechanisms of OR-specific axon guidance mechanisms, it is vital to characterize their expression profiles at the OSN axon termini within the same glomerulus. The aim of this article was to introduce methods for collecting as many glomeruli as possible on a single OB section and for performing immunostaining using multiple antibodies. This would allow the comparison and analysis of the expression patterns of axon-sorting molecules without staining variation between OB sections.
Introduction
During development, neurons are precisely connected with each other to form proper neural circuits, which is critical for the normal brain function. Since aberrant neural circuits in the brain are thought to be the cause of mental disorders such as autism and schizophrenia, understanding the mechanisms of neural circuit formation is one of the major challenges in the field of neuroscience.
In the mouse olfactory system, each Olfactory Sensory Neuron (OSN) in the Olfactory Epithelium (OE) expresses only one functional Olfactory Receptor (OR) gene and OSNs expressing the same OR converge their axons to a specific pair of glomeruli at stereotyped locations in the Olfactory Bulb (OB)1,2. The mouse olfactory system is an excellent model system for studying the molecular mechanisms of neural circuit formation because researchers can utilize the OR expression to identify a specific subtype of OSNs and visualize the projection sites of OSN axons as clear glomerular structures. A remarkable feature of OSN projection is that ORs play instructive roles in projecting OSN axons to the OB3,4,5,6. More specifically, after OSN axons are guided to approximate target regions, they are segregated to form glomerulus in an OR-dependent manner. Previous studies have shown that OR molecules control the expression of axon-sorting molecules, which regulate glomerular segregation7,8. Moreover, accumulating evidence suggests that OR molecules generate the neuronal identity code by a unique combination of axon-sorting molecules9. Thus, to understand the mechanism of OR-dependent glomerular segregation, it is necessary to characterize the expression profiles of axon-sorting molecules in OSNs.
Fluorescent immunostaining is a common method to visualize the expression of specific genes. Since proteins of axon-sorting molecules are predominantly localized to OSN axons, researchers need to use OB sections to characterize their expression patterns in OSNs. Coronal sectioning of the OB has been routinely used for immunostaining. However, this preparation loses the topographic information along the anterior-posterior axis in the same OB section. We therefore developed a parasagittal preparation of the medial side of the OB, which can mount as many surrounding glomeruli as possible on the same OB section. Combined with immunostaining using multiple antibodies, this preparation allows the comparison and analysis of the expression patterns of axon-sorting molecules without staining variation between OB sections.
Furthermore, an immunohistochemical staining method has been presented without post-fixation with PFA and sucrose treatment. This method allows researchers to obtain enough high-quality staining data for multivariable data analysis. The protocols presented here will provide details of powerful methods for researchers who study the olfactory neural circuit formation.
Protocol
All experimental procedures were performed with the approval of the animal experiment ethics committee at the University of Tokyo and according to the University of Tokyo guidelines for the care and use of laboratory animals.
1. Preparation of Solutions
- Prepare 0.01 M Phosphate-Buffered Saline (PBS): add a PBS tablet (0.14 M NaCl, 0.0027 M KCl, 0.010 M PO43-, pH 7.4) to 1 L distilled water and stir at RT until it is completely dissolved.
- Prepare 4% paraformaldehyde (PFA) in PBS: add 4 g of PFA to 100 mL PBS. Stir the solution at 60 °C until it is completely dissolved.
- After dissolving the PFA in PBS, adjust the pH of the solution to 7.4 with 1 N sodium hydroxide (NaOH). Then, cool on ice and filter the solution through a filter paper to remove any particulate matter. Store at 4 °C.
Caution: Take appropriate care when using these reagents. Handle with care and use gloves, safety goggles, and lab coat under a chemical hood.
NOTE: Use fresh 4% PFA solution prepared within 2 d.
- After dissolving the PFA in PBS, adjust the pH of the solution to 7.4 with 1 N sodium hydroxide (NaOH). Then, cool on ice and filter the solution through a filter paper to remove any particulate matter. Store at 4 °C.
- Prepare 0.01 M PBS supplemented with 0.3% Triton X-100 (PBST): add 3 mL of Triton X- 100 to 997 mL of PBS. Stir the solution at RT until it is completely dissolved.
- Prepare 1% and 5% blocking solution: add 10 g of skim milk to 200 mL PBST to prepare 5% blocking solution. Stir at RT until it is completely dissolved. Dilute 5% blocking solution five times with PBST to prepare 1% blocking solution.
2. Preparation of Parasagittal OB Sections
- Use animals between 1-2 weeks of age. These age groups are suitable for the following experiments because glomeruli are clearly visible and the brain can be cut with the skull.
- Anesthetize the animal with an intraperitoneal injection of pentobarbital (50 mg/kg body weight). Perfuse the animal transcardially with ice-cold 4% PFA in PBS. Handle with care and use gloves, safety goggles, and lab coat under a chemical hood.
- After perfusion, cut off the head with scissors and remove the skin carefully. Then, insert a blade of the scissors into the space between the upper and lower teeth and cut horizontally to remove the lower jawbone. Trim away excess tissue with forceps and scissors to leave the olfactory tissue including the OB and OE.
NOTE: Do not remove the skull surrounding the OB as this retains the medial glomeruli aligned vertically straight. - Immerse the olfactory tissue in PBS to remove the air in the nasal cavity. Cut off the posterior part of the brain vertically and place the olfactory tissue on the bottom of the embedding mold with the cutting face down. Fill the mold with Optimal Cutting Temperature (O.C.T.) compound and then immerse the mold into liquid nitrogen.
- After the tissue in the compound is completely frozen, incubate it in the cryostat at -20 °C for 1 h.
- Make serial parasagittal sections (10 µm) of the OB with a cryostat and collect them by sticking to MAS-coated glass slides. After sticking, dry the slides immediately with a blow dryer.
3. Day 1: Quadruple Immunostaining of the OB Slices
- Wash the slides for 5 min with PBS at RT. Repeat 3 times.
- Block the nonspecific binding sites by incubating the slides for 1 h with the 5% blocking solution at RT.
- Incubate the slides O/N with a cocktail of primary antibodies (400 µL each slide) in the 1% blocking solution at RT. Use the following primary antibodies: guinea pig anti-Kirrel2 antibody (1:1000), goat anti-Semaphorin-7A (Sema7A) antibody (1:500), rat anti-OL-protocadherin (OLPC) antibody (1:500), and mouse anti-vesicular glutamate transporter2 (VGLUT2) antibody (1:500).
4. Day 2: Quadruple Immunostaining of the OB Slices
- Discard the primary antibody solution and wash the slides for 5 min with PBST at RT. Repeat 3 times.
- Incubate the slides for 1 h with a cocktail of secondary antibodies (400 µL each slide) in PBS at RT. Use the following secondary antibodies: donkey anti-mouse Alexa Fluor 405 (1:400), donkey anti-goat Alexa Fluor 488 (1:400), donkey anti-guinea pig Alexa Fluor 555 (1:400), and donkey anti-rat Alexa Fluor 647 (1:400).
NOTE: All secondary antibodies should come from the same host species. Choose a different wavelength of fluorescence of secondary antibodies for each primary antibody to ensure no overlap of wavelengths. - Discard the solution and wash the slides for 5 min with PBS at RT. Repeat 3 times.
- Mount coverslips on the slides with the mounting medium. For this, apply 2 drops of the mounting medium on each slide, and then, put a coverslip by removing the air bubbles.
5. Intensity Measurements
- Obtain fluorescent images with a fluorescence microscope. Use the following filter cubes: DAPI filter cube (360/40 nm excitation, 460/50 nm emission, and 400 nm dichroic mirror) for Alexa Fluor 405 signals, GFP filter cube (470/40 nm excitation, 525/50 nm emission, and 495 nm dichroic mirror) for Alexa Fluor 488, TRITC filter cube (545/25 nm excitation, 605/70 nm emission, and 565 nm dichroic mirror) for Alexa Fluor 555, and Cy5 filter cube (620/60 nm excitation, 700/75 nm emission, and 600 nm dichroic mirror) for Alexa Fluor 647.
Note: Adjust the exposure time to obtain fluorescent signals of the image without saturation. - Define the glomerular structure by immunofluorescence signals of VGLUT2. Measure staining intensities of axon-sorting molecules within glomeruli with ImageJ.
6. Data Analysis
- Subtract background signals from the staining intensities of axon-sorting molecules.
- Prepare the expression data matrix, which has columns composed of axon-sorting molecules and rows composed of the glomeruli.
- Perform a principal component analysis (PCA) using the prepared expression dataset. Then, obtain the PCA scores, contribution ratios, and factor loadings of each principal component.
Representative Results
The olfactory glomerular map is formed by initial global targeting and subsequent glomerular segregation of OSN axons1,2. Glomerular segregation is regulated by the adhesive/repulsive axonal interactions mediated by axon-sorting molecules whose expression levels are determined by expressed OR molecules7. The axon-sorting molecules involved in glomerular segregation are expressed in a position-independent mosaic manner in the OB9. In this study, we selected the following genes: Kirrel2, Sema7A, OLPC, BIG-2, and PCDH17. Some of these axon-sorting molecules are expressed in an activity-dependent manner7,8,10.
The quadruple immunostaining method shown here allowed the visualization of the expression patterns of four molecules simultaneously on the same section. These axon-sorting molecules showed position-independent mosaic expressions, but their patterns were not identical (Figures 1A, B). The glomerular structure was defined by fluorescent signals of VGLUT2 (Figure 2A). Axon-sorting molecules (Kirrel2, Sema7A, OLPC) were differentially expressed in each glomerulus; however, VGLUT2, which was used as a glomerular marker, was uniformly expressed among glomeruli (Figures 2B, 2C).
A single glomerulus can be considered as a data unit that is represented by multiple variables. To analyze this multivariate expression data, principal component analysis (PCA) was performed. PCA defines a new coordinate system, so that the first coordinate has the greatest variance datasets and that each succeeding coordinate has the greatest residual variance. The expression datasets were subjected to PCA and plotted in the space of the first and second principal component (PC1 and PC2) (Figure 3A). The contribution ratio of each principal component indicates how much percentage each principal component represents in the total tendency of the variables. The contribution ratios of PC1, PC2, PC3, PC4, and PC5 were 33.4%, 28.2%, 19.8%, 17.3%, and 6.3%, respectively (Figure 3B). PCA also revealed the factor loadings in each principal component. The squared factor loadings indicate how much percentage of the variance in an original variable is explained by a factor. In PC1, the factor loadings of Sema7A, OLPC, and Kirrel2 were positively loaded, whereas those of BIG-2 and PCDH17 were not (Figure 3C). A previous study analyzed the expression of these molecules in the mutant mice deficient for the cyclic nucleotide-gated (CNG) channel gene, which is a component of the olfactory signal transduction11, and showed that the patterns of CNG-dependent changes in the expression levels resemble the factor loadings in PC19. These results suggested that the variety of expressions of these molecules was generated by CNG channel-mediated neural activity.
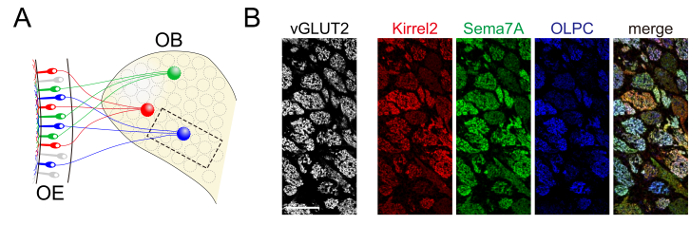
Figure 1: Quadruple Immunostaining of a Parasagittal OB Section. (A) Schematic diagram of the OE & OB. (B) A parasagittal OB section from a 2 week-old mouse immunostained with antibodies against v GLUT2 (white), Kirrel2 (red), Sema7A (green), and OLPC (blue). The merged image of Kirrel2 (red), Sema7A (green) and OLPC (blue) was shown at right. Scale bars = 100 µm. Please click here to view a larger version of this figure.
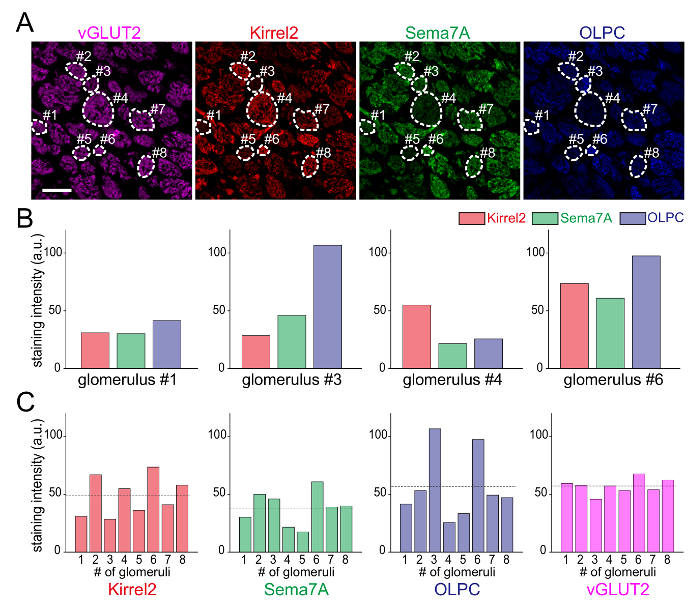
Figure 2: Expression Analysis of Axon-sorting Molecules. (A) A parasagittal olfactory bulb (OB) section immunostained with antibodies against VGLUT2 (purple), Kirrel2 (red), Sema7A (green), and OLPC (blue). Each glomerulus is defined by fluorescent signals of VGLUT2 (pre-synaptic marker). Glomerular structures are surrounded by dashed lines. (B) Expression levels of Kirrel2, Sema7A, and OLPC in glomeruli #1, #3, #4, and #6. Staining intensities of axon-sorting molecules were measured in each glomerulus. (C) Expression levels of the axon-sorting molecules and VGLUT2 compared among the glomeruli. Signal intensities of these molecules were measured in each glomerulus. Grey bars on the graphs indicate the average of expression level. Scale bar = 100 µm. Please click here to view a larger version of this figure.
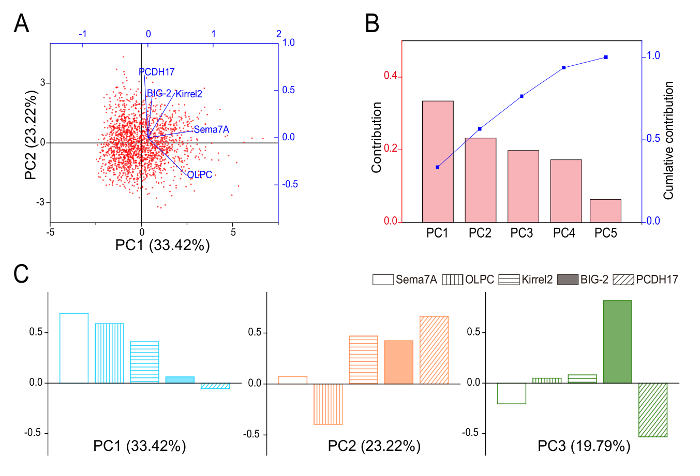
Figure 3: PCA of Expression Data of Axon-sorting Molecules. (A) Principal component analysis (PCA) score biplots (PC1 Vs. PC2) of the expression dataset of five axon-sorting molecules (Kirrel2, Sema7A, OLPC, BIG-2, and PCDH17). A total of 1799 glomeruli were analyzed. (B) The contribution ratios and cumulative contribution ratios of all five principal components. The contribution ratios of PC1, PC2, PC3, PC4, and PC5 are 0.334, 0.282, 0.198, 0.173, and 0.063, respectively. (C) The factor loadings of Kirrel2, Sema7A, OLPC, BIG-2, and PCDH17 in PC1-3. Eigenvalues of PC1, PC2, and PC3 are 1.67, 1.16, and 0.99, respectively. Please click here to view a larger version of this figure.
Discussion
Quadruple immunostaining of parasagittal OB sections enabled the visualization and quantification of the expression levels of as many as four axon-sorting molecules simultaneously in a larger number of glomeruli. By analyzing these multivariable data with PCA, the characteristics for the expression of those molecules can be speculated.
For successful staining, the tissue sample preparation is critically important. Some protocols suggest that tissues should be post-fixed with 4% PFA and treated with 30% sucrose for cryoprotection. However, these steps were omitted in this protocol. This is because, in many cases, these steps reduce the staining signal intensity and increase the background. Moreover, sections should not be dried out at any steps to avoid high background during staining.
The optimal staining condition is different depending on the types of tissues and antibodies used. Therefore, it is necessary to determine the specific staining conditions for each experiment. Furthermore, the concentration of primary antibodies also is important. When the concentration is too high, it increases the background. However, when the concentration is too low, it reduces the signal. It is, therefore, important to identify the appropriate concentration of primary antibodies to obtain high-quality staining results. One limitation is that this method depends significantly on the quality of the primary antibodies and the primary antibody combination with respect to animal species.
Coronal sections of the OB have been routinely used for immunostaining. However, in this protocol, parasagittal sections of the medial side of the OB were used to be able to place more glomeruli on a single section. Since these sections retain the topographic order of the glomeruli along the dorsal-ventral and anterior-posterior axes, they can also be used to analyze the distribution patterns of axon guidance molecules, which determine the projection sites of OSN axons.
In this study, parasagittal sections of the OB were prepared to enable placing more glomeruli on a single section. Furthermore, horizontal sections could be also useful for researchers to reveal the differences along the medial-lateral or anterior-posterior axis. With the development of antibodies that can be used for multiple immunostainings, this quadruple staining method can be applied not only to the OB but also to other brain areas.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Mitsubishi Foundation, the Takeda Science Foundation, JST PRESTO and JSPS KAKENHI Grant Number 16H06144.
Materials
| Phosphate Buffered Saline (PBS) Tablets, pH7.4 | TAKARA BIO | T9181 | |
| Skim Milk | nacalai tesque | 31149-75 | |
| goat anti-Sema7A antibody | R&D Systems | AF2068 | |
| rat anti-OLPC antibody | Merck Millipore | MABT20 | |
| mouse anti-VGLUT2 antibody | Merck Millipore | MAB5504 | |
| goat anti-BIG-2 antibody | R&D Systems | AF2205 | |
| gunea pig anti-Kirrel2 antibody | Operon Biotechnologies | Anti-Kirrel2 antibodies were generated by immunizing guinea pigs with KLH-conjugated synthetic peptides (644-673aa): CRLYRARAGYLTTPHPRAFTSYMKPTSFGP | |
| donkey anti-mouse Alexa Fluor 405 | Abcam | ab175658 | |
| donkey anti-goat Alexa Fluor 488 | Jackson ImmunoResearch | 705-545-003 | |
| donkey anti-guinea pig Alexa Fluor 555 | Thermo Fisher Scientific | A21432 | |
| donkey anti-rat Alexa Fluor 647 | Jackson ImmunoResearch | 712-605-153 | |
| Paraformaldehyde | Wako | 162-16065 | |
| MAS coated slide glasses | MATSUNAMI | MAS-01 | |
| forceps | Fine Science Tools | 11253-27 | |
| Vannas Spring Scissors | Fine Science Tools | 15000-00 | |
| dissecting scissors | Fine Science Tools | 14090-09 | |
| fluorescent microscope | KEYENCE | BZ-X700 | |
| DAPI filter cube | KEYENCE | OP-87762 | |
| GFP filter cube | KEYENCE | OP-87763 | |
| TRITC filter cube | KEYENCE | OP-87764 | |
| Cy5 filter cube | KEYENCE | OP-87766 | |
| filter paper | ADVANTEC | 00011185 | |
| O.C.T compound | Sakura Finetek | M71484 |
References
- Mori, K., Sakano, H. How is the olfactory map formed and interpreted in the mammalian brain?. Annu Rev Neurosci. 34, 467-499 (2011).
- Takeuchi, H., Sakano, H. Neural map formation in the mouse olfactory system. Cell Mol Life Sci. 71, 3049-3057 (2014).
- Mombaerts, P., et al. Visualizing an olfactory sensory map. Cell. 87, 675-686 (1996).
- Feinstein, P., Mombaerts, P. A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell. 117, 817-831 (2004).
- Ishii, T., et al. Monoallelic expression of the odourant receptor gene and axonal projection of olfactory sensory neurones. Genes Cell. 6, 71-78 (2001).
- Nakashima, A., et al. Agonist-independent GPCR activity regulates anterior-posterior targeting of olfactory sensory neurons. Cell. 154, 1314-1325 (2013).
- Serizawa, S., et al. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 127, 1057-1069 (2006).
- Kaneko-Goto, T., Yoshihara, S., Miyazaki, H., Yoshihara, Y. BIG-2 mediates olfactory axon convergence to target glomeruli. Neuron. 57, 834-846 (2008).
- Ihara, N., Nakashima, A., Hoshina, N., Ikegaya, Y., Takeuchi, H. Differential expression of axon-sorting molecules in mouse olfactory sensory neurons. Eur J Neuro. 44, 1998-2003 (2016).
- Williams, E. O., et al. Delta Protocadherin 10 is Regulated by Activity in the Mouse Main Olfactory System. Front Neural Circuits. 5, 9 (2011).
- Brunet, L. J., Gold, G. H., Ngai, J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 17, 681-693 (1996).

