Optimized Management of Endovascular Treatment for Acute Ischemic Stroke
Summary
The outcome of patients with acute ischemic stroke depends on swift restoration of cerebral blood flow. This protocol aims at optimizing the management of such patients by minimizing peri-procedural timings and rendering the time from hospital admission to reperfusion as short as possible.
Abstract
This manuscript describes a streamlined protocol for the management of patients with acute ischemic stroke, which aims at the minimization of time from hospital admission to reperfusion. Rapid restoration of cerebral blood flow is essential for the outcomes of patients with acute ischemic stroke. Endovascular treatment (EVT) has become the standard of care to accomplish this in patients with acute stroke due to large vessel occlusion (LVO). To achieve reperfusion of ischemic brain regions as fast as possible, all in-hospital time delays have to be carefully avoided. Therefore, management of patients with acute ischemic stroke was optimized with an interdisciplinary standard operating procedure (SOP). Stroke neurologists, diagnostic as well as interventional neuroradiologists, and anesthesiologists streamlined all necessary processes from patient admission and diagnosis to EVT of eligible patients. Target times for every step were established. Actually achieved times were prospectively recorded along with clinical data and imaging scores for all endovascularly treated stroke patients. These data were regularly analyzed and discussed in interdisciplinary team meetings. Potential issues were evaluated and all staff involved was trained to adhere to the SOP. This streamlined patient management approach and enhanced interdisciplinary collaboration reduced time from patient admission to reperfusion significantly and was accompanied by a beneficial effect on clinical outcomes.
Introduction
EVT is the standard of care to treat patients with acute ischemic stroke due to LVO1,2,3,4,5. Good collateral status and an early restoration of blood flow in the affected brain region determine clinical outcomes in such patients6,7. Hence, it is crucial to avoid any delay of treatment. The time from symptom onset to hospital admission depends on the individual circumstances of each patient and can hardly be influenced by the treating stroke physicians. Therefore, all hospital-inherent potentially treatment-delaying factors should be carefully avoided. For this, an interdisciplinary SOP has been developed at our hospital in February 2014, which streamlined the process from patient admission to EVT8. Stroke neurologists, diagnostic as well as interventional neuroradiologists, and anesthesiologists established a detailed workflow and defined target times for every step. Actually achieved times were prospectively recorded along with clinical data and imaging scores for all endovascularly treated stroke patients in a comprehensive database approved by the local ethics committee. These data were regularly analyzed and discussed in interdisciplinary team meetings. Potential issues were evaluated and all staff involved was trained to adhere to the SOP.
The analysis of the recorded data revealed a significant reduction of time from patient admission to reperfusion. In addition to that, a beneficial effect on clinical outcomes was observed8. Based on these findings, and on the fact that we could not achieve further time reductions after fully exploiting the potential of measures such as increased sense of urgency and teamwork, the SOP was refined to further reduce in-hospital times by combining imaging and treatment in the angiography suite9.
This refined SOP came into effect in 2016. Patients with suspected stroke, a symptom duration of less than 6 h, and significant functional impairment were determined to have 7 or more points on the National Institutes of Health Stroke Scale (NIHSS) and were treated with this one stop management approach. This cut-off value of 7 points has been chosen because a recent publication identified a NIHSS score of 7 as the best predictor for LVO10. Patients eligible according to the criteria mentioned above are directly transferred to the angiography suite, where a flat panel detector CT (FDCT) is used to depict acute ischemic signs and to exclude intracranial hemorrhage. A biphasic FDCT angiography (FDCTA) is performed to identify LVO. Then, recombinant tissue plasminogen activator (rtPA) is administered intravenously in eligible patients and EVT is carried out immediately after evaluation of the images in the same room.
Preliminary data show a further significant reduction of time from admission to reperfusion compared to other studies proposing a streamlined patient management11. Patients not meeting the criteria required for the one stop management approach, i.e. less severe symptoms (NIHSS below 7) and/or more than 6 h elapsed since symptom onset, are managed following the initial routine including diagnostic imaging with conventional multi-detector CT, CT angiography (CTA), and CT perfusion (CTP).
Here, the optimized interdisciplinary workflow for a swift treatment of patients with acute ischemic stroke is described in detail. The protocol is tailored to a comprehensive stroke center equipped with a latest generation angiography system.
Protocol
Procedural timings, interventional features, and clinical data described in this protocol are derived from a comprehensive observational database, which was approved by the local ethics committee (approval number 4/11/08 and 15/7/13). Patients' consent for treatment was obtained according to common clinical guidelines; the need for a separate consent concerning the inclusion in the database was waived by the ethics committee.
1. Patient Management in the Emergency Room (ER) – Target Time: 10 min
NOTE: The following steps have to be performed by the stroke neurologist.
- Inform the neuroradiologist that a patient with signs suggestive of acute stroke is expected prior to the arrival of patient. State age and symptom onset, if known.
- Furthermore, inform the anesthesiologist about the potential upcoming EVT (first call).
- Have the neuroradiologist inform the interventional neuroradiologist about a suspected upcoming acute stroke patient.
- Perform a rapid clinical assessment of the patient upon arrival, including the quantification of functional impairment according to the NIHSS. Thus, test the level of consciousness, vision, motor and sensory function, language and speech, as well as extinction and inattention.
- Meanwhile, have the ER nurse place two large peripheral venous catheters and take a blood sample for immediate laboratory analysis.
- Have the ER nurse attach ECG, blood pressure, and blood oxygen saturation mobile monitoring systems to the patient.
- If stroke is suspected, escort the patient to the angiography suite (scenario A) or to the CT imaging site adjacent to the angiography suite (scenario B), depending on NIHSS score and time from symptom onset to admission.
- Take a backpack containing emergency equipment and a complete set for intravenous thrombolysis. Include 90 mg rtPA, syringes, i.v. lines, and a syringe pump in this set.
- If the clinical condition of the patient is not consistent with stroke, treat the patient according to respective neurological guidelines.
2. Diagnostic Imaging in Scenario A = NIHSS 7 or Above and Less than 6 h Elapsed since Symptom Onset – Target Time to Imaging: 15 min
NOTE: The following steps have to be performed by the neuroradiologist.
- Have the neurologist escort the patient directly to the angiography suite.
- Position the patient on the angiographic table together with the neurologist and the neuroradiological technician.
- Position the patient’s head within the headholder so that the orbitomeatal line is parallel to the rotation pane. Cover the eyes and fixate the head with two straps to prevent motion.
- Perform a standard 20 s non-enhanced rotational FDCT and a standard biphasic FDCTA.
- Perform FDCT on the angiography system using the following parameters: 20 s rotation; 200° total angle with ~500 projections; 109 kV; 1.8 µGy/frame; effective dose ~2.5 mSv.
- For the FDCTA on the angiography system, perform intravenous injection of 60 mL contrast agent at an injection rate of 5 mL/s, followed by 60 mL saline chaser at the same injection rate of 5 mL/s.
- Use the antecubital vein of the right arm in order to optimize the bolus concentration. Use a power injector for injection.
- Use the following specifications of FDCTA: 2 x 10 s rotation; 200° total angle (0.8° per frame); 70 kV; 1.2 µGy/frame; effective dose ~2.5 mSv.
NOTE: The first rotation is timed after a bolus-watching digital subtraction angiography to capture the peak arterial phase, while the second phase is acquired automatically after 5 s correlating to the venous phase. Raw data are automatically transferred and automatically reconstructed on a commercially available workstation.
- Review the acquired images together with the interventional neuroradiologist to rule out an intracranial hemorrhage using the FDCT and to detect LVO using the early phase of the FDCTA. Use the late venous phase of the FDCTA to evaluate the collateral status.
- After exclusion of an intracranial hemorrhage and confirmation of patient's eligibility, have the neurologist start intravenous administration of rtPA (dose: 0.9 mg/kg infused over 60 min with 10% of the total dose as an initial bolus).
NOTE: Total target time from admission to initiation of rtPA treatment: 20 min. - Call the anesthesiologist (second call) and confirm an upcoming EVT.
- Have mobile monitoring devices replaced by stationary devices present in the angiography suite and start preparations for EVT immediately as described in step 3 of this protocol (see step 3. "Preparation of EVT").
- If FDCT and FDCTA are not consistent with acute ischemic stroke, symptoms sustain, and the patient is eligible for an MRI scan, have the neurologist escort the patient to the MRI suite. Perform an MRI to further investigate the neurological condition of the patient.
3. Diagnostic Imaging in Scenario B = NIHSS below 7 and/or More than 6 h Elapsed since Symptom Onset – Target Time: 25 min
NOTE: The following steps have to be performed by the neuroradiologist.
- Have the neurologist escort the patient to the CT suite.
- Have the neuroradiological technician perform a non-enhanced CT scan immediately after arrival of the patient at the imaging site.
NOTE: One can decide to perform an MRI instead of CT only if CT is not possible or if other factors favor an MRI scan. - Read the CT images quickly in order to rule out an intracranial hemorrhage and a large demarcated infarct.
- After excluding both, confirm the patient's eligibility. If less than 4.5 h have elapsed since symptom onset, have the neurologist start intravenous administration of rtPA (dose: 0.9 mg/kg infused over 60 min with 10% of the total dose as an initial bolus).
- Give the initial bolus on-site while the patient is lying on the CT table.
NOTE: Total target time from admission to initiation of rtPA treatment: 20 min.
- Give the initial bolus on-site while the patient is lying on the CT table.
- Have the neuroradiological technician perform CT angiography (CTA) and perfusion (CTP) scans.
- After completion of CTA and CTP, evaluate whether the patient is eligible for EVT.
NOTE: Eligibility criteria are: presence of LVO on CTA detected by lacking opacification and absence of a large demarcated infarct determined using the Alberta Stroke Program Early CT score (ASPECTS)12 on non-enhanced CT. ASPECTS values of 4 and below indicate large infarct demarcation.- Use CTP to determine the amount of still salvageable tissue. For this determination, assess ASPECTS on maps of cerebral blood volume (CBV); patients are eligible for EVT with a CBV-ASPECTS of above 4.
- If the patient is eligible for EVT, call the anesthesiologist and confirm upcoming EVT.
- Have the neurologist transfer the patient immediately to the adjacent angiography suite, where the patient is positioned on the angiographic table and mobile monitoring devices are replaced by stationary devices present in the angiography suite.
4. Preparation of EVT – Target time: 10 min
NOTE: The following steps have to be performed by the neuroradiologist.
- Prepare the patient for the EVT together with the neuroradiological technician.
- Have the neuroradiological technician setup the material required for EVT, i.e. catheters, flushing infusions, syringes, etc.
- Shave and disinfect the patient's groin with skin antiseptic (e.g., kodan tincture forte) and place sterile drapes to ensure aseptic conditions for EVT.
- Have the anesthesiologist commence conscious sedation: induce with ketamine (1 mg/kg) in combination with propofol (1 mg/kg) intravenously and maintain a continuous infusion of propofol (1-2 mg/kg/h) to ensure sufficient spontaneous breathing and patient cooperation.
NOTE: Conscious sedation is the preferred method during EVT.- If conscious sedation seems inappropriate due to sustained agitation or movement of the patient, have the anesthesiologist intubate the patient, so that EVT is performed under general anesthesia.
- Induce with a sufentanil bolus (0.2 – 0.4 µg/kg) and a propofol bolus (1.5 -2 mg/kg) intravenously. Improve intubation conditions with muscle relaxation using rocuronium boli (0.6 mg/kg). Maintain general anesthesia with sevoflurane (0.5-1.5 MAC) and additional repetitive boli of sufentanil (0.2-0.5 µg/kg), if necessary. To facilitate intubation, sway the angiographic table towards the ventilation machine.
- If conscious sedation seems inappropriate due to sustained agitation or movement of the patient, have the anesthesiologist intubate the patient, so that EVT is performed under general anesthesia.
5. Performance of EVT
NOTE: The following steps have to be performed by the interventional neuroradiologist.
- Puncture the right femoral artery in the groin using an 18 G puncture needle and introduce a peripheral 8F guiding sheath. Then start EVT; total target time from patient admission to groin puncture is 30 min in scenario A and 45 min in scenario B.
- Perform EVT.
- Choose the Stent retriever Assisted Vacuum-locked Extraction (SAVE)13 technique as the primary treatment approach. If needed, adapt the procedure to individual requirements of the situation. In cases of tandem occlusions, use the ReWiSed CARe technique for simultaneous thrombectomy of the intracranial lesion and treatment of the cervical stenosis14.
- Have the anesthesiologist closely monitor the vital signs of the patient during the whole procedure. In particular, have the anesthesiologist take immediate action to prevent hypotension.
- After a control angiogram confirms successful reperfusion, defined as a modified Thrombolysis in Cerebral Infarction (mTICI) score of 2b – 3, perform a flat-panel detector angiographic CT. Read the images to rule-out complications of the treatment, e.g. intracranial hemorrhage.
- Remove all materials and dress the wound in the groin. Use a vascular closure device to close and seal the puncture in the femoral artery.
6. Post EVT Procedures and Follow-up
NOTE: The following steps have to be performed by the neuroradiologist.
- Remove all drapes, position the patient in a bed, and make the patient ready for transport to the Intensive Care Unit (ICU) or Stroke Unit together with the neuroradiological technician.
- Have the anesthesiologist escort the patient to the ICU or Stroke Unit, where the patient is treated according to neurological guidelines.
- Perform a follow-up non-enhanced CT after 24 h or upon clinical deterioration of the patient.
- All clinical data, imaging scores, and achieved times are prospectively recorded in a comprehensive database approved by the local ethics committee.
Representative Results
The streamlined management of patients with acute ischemic stroke as described above and shown in Figure 1 was accompanied by an improvement of peri-procedural times in our hospital. The median time from hospital admission to groin puncture was reduced by approximately half an hour when comparing the year prior to the year after implementation of the first version of the SOP (94 min in 2013 and 65 min in 2014). The revision of the SOP including the one stop management approach implemented in 2016 led to a further reduction of the median time from admission to groin puncture (65 min in 2014 and 45 min in 2016; Figure 2). The comparison of those patients who underwent EVT managed with the one stop approach (Scenario A) to those who were treated endovascularly after the initial approach, including conventional CT imaging (similar to Scenario B), shows that the median time from admission to groin puncture was reduced by slightly more than half if patients were transferred to the angiography suite directly (20.5 min in Scenario A and 54.5 min in Scenario B, Table 1). Also, the total time from hospital admission to reperfusion was significantly shorter when the one stop management approach was applied (65 min in Scenario A and 106 min in Scenario B, Table 1).
As expected, the SOP had no effect on the time from symptom onset to hospital admission (77 min before and 80 min after implementation of the SOP), as the treating stroke team cannot influence this period. The achieved times for individual steps between admission to the hospital and reperfusion before and after the SOP came into effect have been analyzed and compared (Figure 2). The faster supply of imaging (mean time between admission and imaging 31 min prior to, 19 min after the initial, and 9.5 min after the revised SOP) and in particular the prompt transfer of patients eligible for EVT to the angiography suite contributed most to the time-to-treatment reduction observed after implementation of the initial as well as the revised version of the SOP (Figure 2). The duration of EVT itself was also shorter after introduction of the initial SOP in 2014 (median 58 min prior to, and 42 min after the implementation of the initial SOP). This was independent from the devices used during EVT8. The time from groin puncture to reperfusion did not change after the revised SOP came into effect (Figure 2). The functional outcome of patients was significantly better after implementation of the first version of the SOP in 2014 (data have been published in detail elsewhere)8. Both an increase of patients with no residual functional impairment, determined by having a value of 0 on the modified Ranking Scale (mRS) (1.5% before and 9.1% after the SOP), and a general shift towards lower degrees of remaining disability was observed after workflow optimization (ordered logistic regression analysis: OR 0.56; 95% CI 0.32-0.98; p = 0.038)8.
Even though a NIHSS score of 7 points or above provides a gross estimate of the presence of LVO10, the symptoms of the patient could clearly originate from other pathologies. An observational analysis of the first 30 patients managed with the described one stop approach provides an indication of the frequency of findings other than ischemic stroke due to LVO on FDCT and FDCTA. An intracranial hemorrhage was detected in 13% (4/30) of patients and an occlusion of a peripheral vessel in 7% (2/30) of patients. Another 13% (4/30) of patients showed neither hemorrhage, vessel occlusion, nor other pathologies, and were diagnosed with Todd's paresis15.
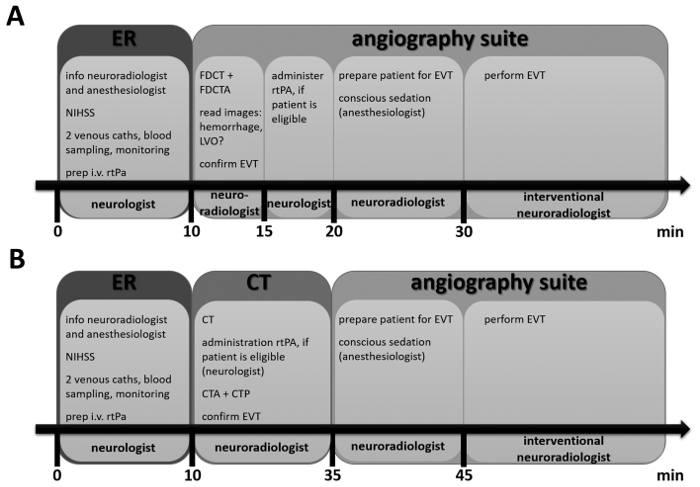
Figure 1: Timeline for Scenario A and for Scenario B. These timelines provide an overview of the steps that are performed, when a patient with suspected acute stroke is admitted to the hospital. The locations are indicated by larger boxes and the person with main responsibility for each step is indicated at the bottom. The smaller inserted boxes list actions important in each step. Details can be found in the protocol. Please click here to view a larger version of this figure.
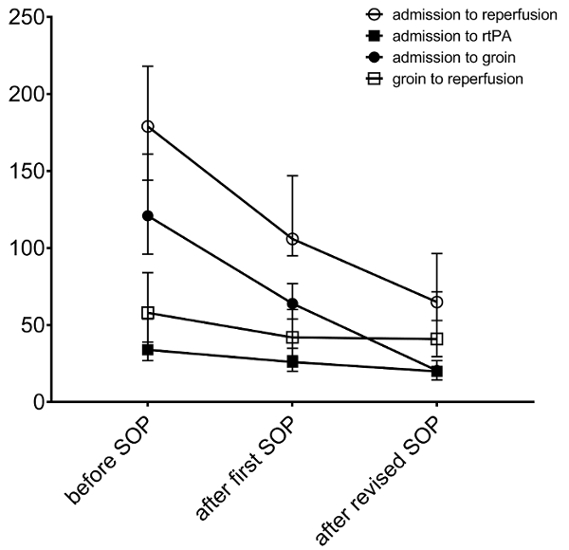
Figure 2: Representative peri-procedural times achieved with process optimization. Median times (in min) for individual steps between symptom onset and reperfusion before and after introduction of the initial as well as the revised SOP are shown. The error bars represent interquartile ranges. Please click here to view a larger version of this figure.
| door to groin (min) | door to reperfusion (min) | |||
| one stop approach (Scenario A) | conventional approach (similar to Scenario B) | one stop approach (Scenario A) | conventional approach (similar to Scenario B) | |
| n | 30 | 44 | 30 | 44 |
| Median | 20.5 | 54.5 | 65 | 106 |
| IQR | 17–27 | 44–66 | 53–96.5 | 88.5–121.5 |
| p-value | <0.001 | <0.001 | ||
Table 1: Median time from hospital admission to groin puncture and reperfusion in 2016. Scenario A consists of a one stop management approach where eligible patients (severe symptoms as determined with an NIHSS score of 7 and above and admitted to the hospital within 6 h of symptom onset) are directly transferred to the angiography suite. Imaging and EVT are performed in the same place. These patients were compared to otherwise matched (regarding NIHSS, symptom-to-door time, availability of the angiography suite) patients that were managed following the workflow implemented with the first version of the SOP. This management was similar to Scenario B and included diagnostic imaging with non-enhanced CT, CTA, and CTP. IQR, interquartile range. Statistical significance was tested with a Mann-Whitney-U test and significance level was set to α = 0.05.
Discussion
This protocol streamlines the management of patients with acute ischemic stroke effectively, which leads to a significant reduction of process times. Interdisciplinary teamwork and communication are crucial for the success of this procedure. Regular team meetings including a review of achieved process times and the discussion of problems and potential solutions are important. All neuroradiologists, technicians, neurologists, anesthesiologists, and nurses involved have to be regularly trained to maintain good performance. Regular meetings and training should also focus on sustaining an increased awareness of the importance of swift reperfusion. It is conceivable that an increased sense of urgency could also have influenced EVT itself, as EVT duration was shorter after implementation of the first version of the SOP, independently from the devices used8. Potentially, the increased awareness of how important a swift reperfusion is for clinical outcomes motivated all the staff involved in EVT to perform the necessary steps faster. However, the effects of increased awareness are difficult to measure.
Primary imaging modalities used for the detection of early ischemic changes and the exclusion of intracranial hemorrhage in the proposed protocol are, respectively, FDCT and conventional CT. FDCTA and CTA, respectively, are used to identify LVO and to evaluate collateral status. However, the protocol can be modified so that patients who are not eligible for the one stop management approach receive a cerebral MRI scan for diagnosis. In addition to that, the 6 h cut-off value for the one stop management approach could be extended into the future. Preliminary results from the "Diffusion Weighted Imaging (DWI) or Computerized Tomography Perfusion (CTP) Assessment With Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention" (DAWN) trial19 suggest that selected stroke patients could benefit from EVT even though they were admitted to the hospital more than 6 hours after symptom onset20. Results from the currently ongoing "Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3" (DEFUSE 3) trial, which investigates EVT performed in patients 6–16 h after stroke onset, could provide further insight on this issue.
The SOP is designed for a comprehensive stroke center equipped with a latest generation angiography system allowing for high quality FDCT imaging and EVT. Primary stroke centers without the ability to perform EVT can follow the protocol according to Scenario B. If intracranial hemorrhage has been excluded with conventional non-enhanced CT, the administration of rtPA should be started in the primary stroke center. Then, the patient should be transferred to a comprehensive stroke center for EVT immediately under ongoing rtPA-therapy ("drip-and-ship").
The proposed protocol has some limitations. First, the reliable exclusion of hemorrhagic stroke with FDCT is required for implementing a one stop management approach. In the past, inaccurate detection of intracranial hemorrhage was the biggest hurdle in using FDCT for stroke diagnosis21,22. This situation seems to be improved when FDCT is performed with the latest generation of angiography systems23. Leyhe et al. reported not only high sensitivity and specificity for the detection of intracranial hemorrhage, but also demonstrated the feasibility of gray-white differentiation in the supratentorial region with the latest generation of FDCT24. However, the possibility of detecting infratentorial bleeding or perimesencephalic subarachnoidal hemorrhage with FDCT is still limited due to beam hardening artifacts and the low soft tissue resolution of FDCT25. Hence, a neuroradiologist with experience in evaluating FDCT images should carefully review the images for absence of intracranial hemorrhage and ultimately clear the patient for rtPA treatment. Considering these aspects, the proposed one stop management is limited to hospitals equipped with a latest generation angiography system and with staff experienced in interpretation of FDCT and FDCTA always available. Otherwise, the sole use of FDCT and FDCTA for reliably excluding hemorrhage and determining large artery occlusion carries the risk of misdiagnosis. Another limitation of FDCTA compared to a conventional CTA is that it covers the extracranial vessels to a lesser extent. While the extracranial carotid artery and the carotid bifurcation are covered, and can be evaluated, the aortic arch is not included at the moment, but will be in the future. The decreased door to reperfusion times we observed show that this potential issue does not lead to any major delays during the intervention. Finally, the protocol is tailored to the conditions in our hospital and may not work equally well in different settings. However, we think that a similar one stop approach can be implemented in other hospitals, despite structural differences.
Fast reperfusion is crucial for the outcome of patients with acute ischemic stroke. Every 30 min delay in time to reperfusion reduces the likelihood of achieving an independent level of functioning by 10%26. A recent meta-analysis of the five randomized trials that demonstrated the benefits of EVT showed that earlier treatment with EVT plus medical treatment was associated with a better outcome compared to medical treatment alone6. Hence, the Stroke Treatment Academic Industry Roundtable included the optimization of patient management in order to reduce time from hospital admission to reperfusion as one priority for future research in EVT27. Moreover, the Society of Neurointerventional Surgery suggested ideal time metrics for stroke processes28. The median time from hospital admission to groin puncture achieved with the revised SOP described above was within the suggested ideal of <60 min. Additionally, the median time from admission to reperfusion for patients managed with the one stop approach is largely within the ideal of <90 min. However, this ideal process time was not met in patients managed with the initial approach including conventional CT, CTA and CTP, as the median time from admission to reperfusion was 106 min in this subgroup.
As the initial observations of a significant reduction of time hospital from admission to reperfusion with the streamlined protocol above are promising, a larger prospective trial to further evaluate this approach is currently being planned.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| Actilyse (recombinant tissue plasminogen activator) | Boehringer Ingelheim, Ingelheim am Rhein, Germany | n/a | generic products from other pharmaceutical companies can be used |
| Imeron 400 (contrast agent) | Bracco Imaging GmbH, Konstanz, Germany | n/a | generic products from other companies can be used |
| Siemens ArtisQ angiography system | Siemens Healthcare, Forchheim, Germany | n/a | an angiography system of another manufacturer can be used; specifications of FDCT and FDCTA described in protocol are valid for ArtisQ |
| Siemens syngo X worklplace | Siemens Healthcare, Forchheim, Germany | n/a | a workstation from another manufacturer can be used |
| ketamine (e.g. Ketanest) | Pfizer Pharma PFE GmbH, Berlin, Germany | n/a | generic products from other pharmaceutical companies can be used |
| propofol (e.g. Propofol-Lipuro) | B. Braun Melsungen AG, Melsungen, Germany | n/a | generic products from other pharmaceutical companies can be used |
| sufentanil (e.g. Sufenta) | Janssen-Cilag GmbH, Neuss, Germany | n/a | generic products from other pharmaceutical companies can be used |
| rocuroniumbromid | B. Braun Melsungen AG, Melsungen, Germany | n/a | generic products from other pharmaceutical companies can be used |
| sevoflurane (e.g. Sevofluran) | Baxter Deutschland GmbH Medication Delivery, Unterschleissheim, Germany | n/a | generic products from other pharmaceutical companies can be used |
| vascular closure device (e.g. Angio-Seal) | Terumo Interventional Systems, Eschborn, Germany | n/a | generic products from other companies can be used |
| peripheral 8F guiding sheath | Terumo Interventional Systems, Eschborn, Germany | n/a | generic products from other companies can be used |
| skin antiseptic (e.g. kodan tincture forte, coloured) | Schuelke & Mayr GmbH, Norderstedt, Germany | n/a | generic products from other companies can be used |
| 18 G intradyn puncture needle | B. Braun Melsungen AG, Melsungen, Germany | n/a | generic products from other companies can be used |
References
- Berkhemer, O. A., et al. A randomized trial of intraarterial treatment for acute ischemic stroke. NEJM. 372 (1), 11-20 (2015).
- Campbell, B. C., et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. NEJM. , (2015).
- Goyal, M., et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. NEJM. , (2015).
- Jovin, T. G., et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. NEJM. 372 (24), 2296-2306 (2015).
- Saver, J. L., et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. NEJM. 372 (24), 2285-2295 (2015).
- Sheth, S. A., Liebeskind, D. S. Collaterals in endovascular therapy for stroke. Curr Opin Neurol. 28 (1), 10-15 (2015).
- Saver, J. L., et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 316 (12), 1279-1288 (2016).
- Schregel, K., et al. Effects of Workflow Optimization in Endovascularly Treated Stroke Patients – A Pre-Post Effectiveness Study. PloS One. 11 (12), e0169192 (2016).
- Psychogios, M. -. N., Bähr, M., Liman, J., Knauth, M. One Stop Management in Acute Stroke: First Mothership Patient Transported Directly to the Angiography Suite. Clin Neuroradiol. , (2017).
- Heldner, M. R., et al. Clinical prediction of large vessel occlusion in anterior circulation stroke: mission impossible?. J Neurol. 263 (8), 1633-1640 (2016).
- Frei, D., et al. A standardized neurointerventional thrombectomy protocol leads to faster recanalization times. J Neurointervent Surg. , (2016).
- Pexman, J. H., et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR. Am J Neuroradiol. 22 (8), 1534-1542 (2001).
- Maus, V., et al. Maximizing First-Pass Complete Reperfusion with SAVE. Clin Neuroradiol. , (2017).
- Behme, D., Knauth, M., Psychogios, M. -. N. Retriever wire supported carotid artery revascularization (ReWiSed CARe) in acute ischemic stroke with underlying tandem occlusion caused by an internal carotid artery dissection: Technical Note. Interv Neuroradiol. , 1591019917690916 (2017).
- Psychogios, M. N., Behme, D., et al. One stop management of acute stroke patients – minimizing door to reperfusion times. European Stroke Conference. 26th Conference, Berlin, Germany, May 24-26, 2017: Abstract e-Book. Cerebrovascular Diseases. 43 (Suppl 1), (2017).
- Goyal, M., Almekhlafi, M. A. Dramatically reducing imaging-to-recanalization time in acute ischemic stroke: making choices. AJNR. Am J Neuroradiol. 33 (7), 1201-1203 (2012).
- Sheth, K. N., et al. Advanced modality imaging evaluation in acute ischemic stroke may lead to delayed endovascular reperfusion therapy without improvement in clinical outcomes. J Neurointer Surg. 5, i62-i65 (2013).
- Psychogios, M. N., et al. Alberta Stroke Program Early CT Scale evaluation of multimodal computed tomography in predicting clinical outcomes of stroke patients treated with aspiration thrombectomy. Stroke. 44 (8), 2188-2193 (2013).
- Jovin, T. G., et al. Diffusion-weighted imaging or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with Trevo (DAWN) trial methods. Int J Stroke. 12 (6), 641-652 (2017).
- Jovin, T. G. Late Breaking Abstracts. European Stroke Journal. 2 (Suppl 1), 477-495 (2017).
- Psychogios, M. N., Buhk, J. H., Schramm, P., Xyda, A., Mohr, A., Knauth, M. Feasibility of angiographic CT in peri-interventional diagnostic imaging: a comparative study with multidetector CT. AJNR. Am J Neuroradiol. 31 (7), 1226-1231 (2010).
- Struffert, T., et al. Visualisation of intracerebral haemorrhage with flat-detector CT compared to multislice CT: results in 44 cases. Eur Radiol. 19 (3), 619-625 (2009).
- Frölich, A. M., Buhk, J. -. H., Fiehler, J., Kemmling, A. Voxel-Based Sensitivity of Flat-Panel CT for the Detection of Intracranial Hemorrhage: Comparison to Multi-Detector CT. PloS One. 11 (11), e0165794 (2016).
- Leyhe, J. R., et al. Latest generation of flat detector CT as a peri-interventional diagnostic tool: a comparative study with multidetector CT. J Neurointerv Surg. , (2016).
- Eckert, M., Gölitz, P., Lücking, H., Struffert, T., Knossalla, F., Doerfler, A. Optimized Flat-Detector CT in Stroke Imaging: Ready for First-Line Use?. Cerebrovasc Dis. 43 (1-2), 9-16 (2017).
- Khatri, P., et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. The Lancet. Neurology. 13 (6), 567-574 (2014).
- Jovin, T. G., Albers, G. W., Liebeskind, D. S., STAIR IX Consortium, Stroke Treatment Academic Industry Roundtable: The Next Generation of Endovascular Trials. Stroke. 47 (10), 2656-2665 (2016).
- McTaggart, R. A., et al. Initial hospital management of patients with emergent large vessel occlusion (ELVO): report of the standards and guidelines committee of the Society of NeuroInterventional Surgery. J Neurointerv Surg. 9 (3), 316-323 (2017).

