Synthesis and Mass Spectrometry Analysis of Oligo-peptoids
Summary
A protocol is described for the manual synthesis of oligo-peptoids followed by sequence analysis by mass spectrometry.
Abstract
Peptoids are sequence-controlled peptide-mimicking oligomers consisting of N-alkylated glycine units. Among many potential applications, peptoids have been thought of as a type of molecular information storage. Mass spectrometry analysis has been considered the method of choice for sequencing peptoids. Peptoids can be synthesized via solid phase chemistry using a repeating two-step reaction cycle. Here we present a method to manually synthesize oligo-peptoids and to analyze the sequence of the peptoids using tandem mass spectrometry (MS/MS) techniques. The sample peptoid is a nonamer consisting of alternating N-(2-methyloxyethyl)glycine (Nme) and N-(2-phenylethyl)glycine (Npe), as well as an N-(2-aminoethyl)glycine (Nae) at the N-terminus. The sequence formula of the peptoid is Ac-Nae-(Npe-Nme)4-NH2, where Ac is the acetyl group. The synthesis takes place in a commercially available solid-phase reaction vessel. The rink amide resin is used as the solid support to yield the peptoid with an amide group at the C-terminus. The resulting peptoid product is subjected to sequence analysis using a triple-quadrupole mass spectrometer coupled to an electrospray ionization source. The MS/MS measurement produces a spectrum of fragment ions resulting from the dissociation of charged peptoid. The fragment ions are sorted out based on the values of their mass-to-charge ratio (m/z). The m/z values of the fragment ions are compared against the nominal masses of theoretically predicted fragment ions, according to the scheme of peptoid fragmentation. The analysis generates a fragmentation pattern of the charged peptoid. The fragmentation pattern is correlated to the monomer sequence of the neutral peptoid. In this regard, MS analysis reads out the sequence information of the peptoids.
Introduction
Peptoids are a class of sequence-controlled polymers with backbone structures mimicking the structure of peptides. Peptoids can be synthesized from diverse amines, which enables peptoids to exhibit highly tunable properties1,2. Peptoids have been used as molecular models for biophysical research, considered as therapeutic agents, and designed as ligands for proteins3,4,5,6. Peptoids have been developed into a variety of biologically active compounds, such as anti-fouling and antibody-mimetic materials, antimicrobial agents, and enzyme inhibitors7,8,9. With a highly ordered and tunable nature, peptoids have also been thought of as a type of molecular information storage10. The discovery of these diverse applications calls for the development of efficient analytical methods to characterize the sequence and structure of peptoids. Tandem mass spectrometry-based techniques have shown promise as the method of choice for analyzing the sequence properties of sequence-controlled polymers, including peptoids11,12,13,14,15. However, systematic studies correlating the peptoid ion fragmentation patterns resulting from mass spectrometry studies and the structural information of peptoids are very limited.
Peptoids can be readily synthesized using a solid phase method. The well-developed method involves an iteration of a two-step monomer addition cycle16,17. In each addition cycle, a resin-bound amine is acetylated by a haloacetic acid (typically bromoacetic acid, BMA), and this is followed by a displacement reaction with a primary amine. Although automated synthesis protocols have been routinely applied for peptoid synthesis, peptoids can be synthesized manually with excellent yields in a standard chemistry laboratory16,18,19,20.
Our lab has adopted the method of manual peptoid synthesis and simplified the apparatus used in the existing methods. We have previously studied the fragmentation patterns of a series of peptoids using MS/MS techniques21,22,23. Our results show that peptoids produce characteristic fragmentations when they are subjected to collision-induced dissociation (CID)21,23 or electron-capture dissociation (ECD)22 experiments. In this article, we demonstrate how oligo-peptoids can be synthesized in a standard chemistry laboratory, how to perform the CID experiments using a triple-quadrupole mass spectrometer, and how to analyze the spectral data. The peptoid to be synthesized and characterized is a nonamer with N-terminal acetylation and C-terminal amidation, Ac-Nae-(Npe-Nme)4-NH2. The structure of the peptoid is shown in Figure 1.
Protocol
1. Synthesis of Peptoid
NOTE: The synthesis begins with activating the resin by swelling the resin and removing the protecting group. This is followed by growing the peptoid chain onto the resin through repeating monomer addition cycles. The first monomer coupled to the resin is the C-terminal residue. The peptoid is elongated from the C-terminus to the N-terminus. Once the desired peptoid sequence is achieved, the resin is cleaved off and the peptoid product is purified.
- Preparation of reagents
NOTE: The liquid reagents are measured using a micropipette and the solid reagents are measured using an analytical balance.- Mix 6.2 mL of N, N'-diisopropylcarbodiimide (DIC) and 43.8 mL of N,N-dimethylformamide (DMF) to prepare a 0.8 M of DIC/DMF solution.
- Dissolve 5.56 g of BMA into 50.0 mL of DMF to prepare a 0.8 M BMA/DMF solution.
- Mix 2 mL of piperidine (Pip) and 8 mL of DMF to achieve 20% Pip/DMF solution.
- Dissolve 1.9 mL of Npe into 13.1 mL of DMF to achieve 1.0 M Npe/DMF.
- Dissolve 1.3 mL of Nme into 13.7 mL DMF to achieve 1.0 M Nme/DMF.
- Dissolve 0.32 mL of Nae into 2 mL of DMF to achieve 1.0 M Nae/DMF.
Caution: Most of the chemicals used in the synthesis are hazardous. Trifluoroacetic acid (TFA), DIC, Pip, and BMA are hazardous to the skin, eyes, and respiratory tract. DMF and dichloromethane (DCM) are suspected carcinogens. All reactions should be performed in a fume hood and appropriate personal protective equipment should be used. Please check the material safety data sheet (MSDS) of all chemicals used in the synthesis.
- Resin activation
- Measure out 84 mg of Rink amide resin (Resin, 0.047 mmol, loading 0.56 mmol/g) and add it to a 10 mL polypropylene solid-phase reaction vessel. Insert the plunger into the vessel.
- Add 2 mL of DMF to the reaction vessel and cap the vessel with a pressure cap. Place the vessel on a shaker, and agitate the vessel at room temperature at an angle of movement of approximately 12 degrees and 385 oscillations/min for 30 min. Drain the solution to a waste container by removing the cap and pushing the plunger of the reaction vessel.
- Add 2 mL of 20% Pip/DMF solution to the vessel and cap the vessel. Agitate it on the shaker for 2 min, and drain the solution to the waste container.
- Add 2 mL of 20% Pip/DMF solution to the vessel, cap the vessel, and agitate it at room temperature for 12 min. Remove the cap and drain the solution to the waste container.
- Wash the resin by adding 1 mL of DMF, capping the vessel, and agitating the vessel for 1 min. Remove the cap and drain the solution by pushing the plunger. Wash the Resin with DMF for 4 additional times.
- Monomer addition and N-terminal acetylation
NOTE: Each monomer addition cycle involves two reaction steps, bromoacetylation and displacement.- Carry out the first monomer addition cycle to form Nme-Resin.
- Perform a bromoacetylation reaction. Mix 1 mL of 0.8 M BMA/DMF solution and 1 mL of 0.8 M DIC/DMF solution in a beaker. Transfer the mixture to a reaction vessel containing Resin and cap the vessel. Place the vessel on the shaker and agitate it at room temperature for 20 min. Remove the cap and drain the solution to the waste container.
- Wash the resin by adding 1 mL of DMF, capping the vessel, agitating it for 1 min, and draining the solution by pushing the plunger. Wash the resin by adding 1 mL of DCM, agitating the vessel for 1 min, and draining the solution. Wash the resin with DCM once more, and then wash it with DMF twice.
- Perform a displacement reaction. Add 1 mL of 1.0 M Nme/DMF solution, cap the vessel, agitate the vessel at room temperature for 60 min. Remove the cap and drain the solution by pushing the plunger.
- Wash the resin by adding 1 mL DMF, agitating the vessel for 1 min, and draining the solution by pushing the plunger. Wash the resin by drawing in 1 mL DCM, agitating for 1 min, and draining the solution. Wash DCM once more, followed by washing with DMF twice.
- Repeat monomer addition cycles from steps 1.3.2 to 1.3.5 to form Nae-(Npe-Nme)4-resin. When repeating the step of displacement reaction (1.3.4), use a specific amine solution according to the peptoid sequence.
NOTE: The peptoid chain is elongated from the C-terminus to the N-terminus with the C-terminal residue bound to the Resin. - Perform N-terminal acetylation to form Ac-Nae-(Npe-Nme)4-resin.
- Mix 92 µL of acetic anhydride, 43.5 µL of N,N-diisopropylethylamine (DIPEA), and 2 mL of DMF in a beaker to make about 2 mL of acetylation cocktail.
- Add 2 mL of acetylation cocktail to the vessel containing resin, cap the vessel, and agitate it at room temperature for 60 min. Remove the cap and drain the solution by pushing the plunger.
- Wash the resin by adding 1 mL of DMF, agitating the vessel for 1 min, and draining the solution by pushing the plunger. Wash the resin by adding 1 mL of DCM, agitating the vessel for 1 min, and draining the solution. Repeat washing the resin with DCM once more, then wash with DMF twice more.
- Wash the resin by adding 1 mL of DCM, agitating the vessel for 1 min, and draining the solution by pushing the plunger. Repeat washing with DCM twice. Remove the cap and let the resin air-dry in the reaction vessel for 10 min.
- Cleavage and purification
- Mix 3.8 mL of TFA, 100 µL of triisopropylsilane (TIPS), and 100 µL of HPLC-grade H2O in a beaker to make 4 mL of cleavage cocktail.
- Add 4 mL of freshly made cleavage cocktail to the vessel containing resin, cap the vessel, and agitate it at room temperature for 2 h.
- Remove the cap and collect the filtrate solution into a 50 mL polypropylene centrifuge tube. Add 1 mL of TFA to the vessel, cap it, and agitate for 1 min. Collect the filtrate solution into the same centrifuge tube.
- Evaporate TFA by blowing in a stream of nitrogen gas gently until about 1 mL viscous solution is left.
- Add 15 mL of diethyl ether to the remaining solution, cap the centrifuge tube, and incubate it in a -20 °C freezer for 2 h to overnight. The crude peptoid precipitates as white solid.
- Pellet the solid using a centrifuge at 4,427 x g for 10 min. Remove the cap of the centrifuge tube and decant diethyl ether into a beaker carefully without losing the solid.
Caution: Diethyl ether is a flammable organic solvent. Please use a diethyl ether safe centrifuge. - Wash the solid by adding 10 mL of ice-cold diethyl ether into the centrifuge tube containing the solid, capping the tube, and placing it in the centrifuge. Perform centrifugation at 4,427 x g for 10 min. Remove the centrifuge tube from the centrifuge and decant diethyl ether into a beaker carefully without losing the solid.
- Dry the solid by gently blowing in a stream of nitrogen gas.
- Add 10 mL of HPLC-grade H2O to dissolve the dried solid. Pass the solution through a nylon syringe filter with pore size of 0.45 µm, and collect the filtrate into a pre-weighted 50 mL polypropylene centrifuge tube.
- Shell freeze the solution by placing and rotating the centrifuge tube containing the peptoid solution in a 12-ounce, double-stacked expanded polystyrene cup 1/3 filled with liquid nitrogen. Lyophilize the frozen solution overnight to yield solid peptoid.
- Repeat lyophilization one more time by dissolving the solid peptoid into 10 mL of HPLC-grade H2O, shell freezing in liquid nitrogen, and lyophilizing overnight. The resulting peptoid is sufficiently pure for sequence analysis by mass spectrometry.
2. MS Measurements and Sequence Analysis
NOTE: The MS/MS experiment is carried out in a triple-quadrupole mass spectrometer coupled to an electrospray ionization (ESI) source. Data collection is controlled by using the data acquisition software accompanied with the instrument. The general procedure includes 1) performing the full scan mass spectrometry experiment and recording the mass spectrum, 2) performing the CID MS/MS experiment and recording the MS/MS spectrum, and 3) comparing the MS/MS spectral data with theoretical fragmentation scheme predicted based on the structural feature of the peptoid.
- Preparation of sample solution
- Weigh out 1.0 – 2.0 mg solid peptoid in a 3 – 5 mL glass vial. Add 1 mL mixed solvent of acetonitrile and water (ACN/H2O, 1:1, v/v) to dissolve the peptoid. This gives the stock sample solution with a concentration of about 10-3 M.
- Transfer 20 µL of stock solution into a 1.5 mL centrifuge tube and add 1 mL mixed solvent of ACN/H2O to yield a diluted sample solution of about 10-5 M.
- Remove any possible insoluble particles in the diluted sample solution by performing centrifugation at 4,427 x g for 3 min. Transfer about 700 µL of the top portion of the solution into another 1.5 mL centrifuge tube to make the peptoid MS working solution with concentration of about 10-5 M.
- Adjust the concentration of the MS working solution based on the observed signal intensity during mass spectrometry measurements.
- An alternative way to remove any possible insoluble particles in the diluted sample solution is to pass the sample solution through a 0.20 µm syringe filter and collect the filtrate into a 1.5 mL centrifuge tube to make the peptoid MS working solution of about 10-5 M.
- Recording mass spectra
- Run mixed solvent of ACN/H2O (1:1, v/v) through the electrospray ionization (ESI) source and set up the instrument in a standard positive ion, full scan mass spectrometry mode with a mass-to-charge ratio (m/z) range of 100 – 1500.
Note:
Typical operating parameters:
ESI needle voltage, 5 kV
Capillary voltage, 40 V
Drying gas (nitrogen gas) temperature, 200 °C. - Add approximately 300 µL of peptoid MS working solution (about 10-5 M) into a 500 µL or a 1 mL syringe and connect the syringe to the ESI inlet using capillary polyether ether ketone (PEEK) tubing. Place the syringe onto the syringe pump and set the flow rate at 10 µL/min to infuse the sample solution into the ESI inlet.
- Turn on the ESI needle voltage to activate the ESI process, and then turn on the detector. Set the display in profile mode and the m/z range of 100 – 1,500. View a mass spectrum profile shown in the profile window. The peak at m/z 1,265 (displayed as m/z of 1,264.6) corresponds to the peptoid ion or protonated peptoid.
- Record the MS spectrum for 2 min. In the "Method Window", use 2 min as the "run time." Open the recording window and fill in a proper file name, and start to record the spectrum.
NOTE: The resulting mass spectrum is shown in Figure 3. - Optimize the intensity of the peak at m/z of 1,265. Set the m/z range to 1,150 – 1,350 and adjust the capillary voltage while viewing the peak intensity in mV shown in the profile window. For example, increase the capillary voltage to 50 V or higher and view the change of the peak intensity. The optimal peak intensity is around 150 – 200 mV.
NOTE: Adjusting the capillary voltage can significantly change the abundance of certain ions. Increasing the capillary voltage may enhance the intensity of the peptoid ion. However, a high capillary voltage may also induce dissociation of the peptoid ion in the ion source, and decrease the observed intensity. Adjusting the temperature of drying gas may enhance the intensity of the peak at m/z 1,265, but it is less effective than adjusting the capillary voltage. If the peak at m/z 1,265 does not reach the optimal intensity, adjust the sample concentration (or example, double the concentration of the MS working solution). - Switch the instrument to the MS/MS mode. Set the precursor ion at m/z 1,265 and the MS/MS mass range at m/z of 100 – 1,400. In the "Method Window," use m/z 1,265 as the "Q1 First Mass" and leave the "Q1 Last Mass" blank. Use m/z 100 as the "Q3 First Mass" and m/z 1,400 as the "Q3 Last Mass."
NOTE: In the MS/MS mode, the first quadrupole unit (Q1) functions as a mass filter to isolate the peptoid ion as the precursor ion, the second quadrupole unit (Q2) is a collision cell, and the third quadrupole unit (Q3) functions as a mass analyzer. - Set the collision energy at 40 eV and the collision gas (argon, in this case) pressure at 1.5 mTorr.
- View a mass spectrum profile displayed in the profile window. The peak at m/z of 1,265 corresponds to the peptoid ion, and the peaks with lower m/z values represent the fragments from the peptoid ion.
- Adjust the collision energy to optimize the display of the fragmentation spectrum. For example, increase the collision energy to 45 eV and view the change of the spectrum profile.
NOTE: In general, increasing the collision energy will enhance the abundance of the fragment ions and reduce the abundance of the peptoid ion. Increasing the collision gas pressure will also enhance the abundance of the fragment ions.
Caution: Do not increase the collision gas pressure beyond 2 mTorr. - Record the MS/MS spectrum for 2 min. In the "Method Window," use 2 min as the "run time." Open the recording window and fill in a proper file name, and start to record the spectrum.
- Repeat recording 1 – 2 times.
- Run mixed solvent of ACN/H2O (1:1, v/v) through the electrospray ionization (ESI) source and set up the instrument in a standard positive ion, full scan mass spectrometry mode with a mass-to-charge ratio (m/z) range of 100 – 1500.
- Peptoid sequence analysis
NOTE: Under the CID condition, the peptoid ion would fragment at the amide bonds along the peptoid backbone to produce a series of N-terminal fragments called B-ions, and a series of C-terminal fragments called Y-ions- Draw out the chemical structure of the acetylated peptoid by placing the N-terminus on the left side and the C-terminus on the right side, and draw a dashed line at each amide bond to generate a fragmentation scheme as shown in Figure 2a. Draw a proton with a dashed circle to indicate the charge carrier. Starting from the left side of the structure, place labels on the dashed lines to indicate the N-terminal fragments as B1, B2, to B8. Starting from the right side, place labels on the dashed lines to indicate the C-terminal fragments as Y1, Y2, to Y8.
NOTE: A chemical drawing software can be utilized to draw the peptoid structure. - Imagine fragmentation at the 4th amide bond from the left side, and draw the structures of the N-terminal fragment and the C-terminal fragment with proper formal charges, as shown in Figure 2b. Place the label of B4 on the N-terminal fragment, and place the label of Y5 on the C-terminal fragment. Calculate the m/z value of B4 by summing up the nominal masses of the elements in the structure to yield a value of 580, and place m/z 580 on the N-terminal fragment. Calculate the m/z value of Y5 and place m/z 685 on the C-terminal fragment.
- Calculate the m/z values for all eight B ions and all eight Y ions, and assemble them in a table, as shown in Table 1.
NOTE: The m/z values of the fragments can be calculated using a chemical drawing software. - Open the recorded MS/MS spectrum of the peptoid using the data review software accompanied with the instrument. Set the Labeling Preferences to "Show Ion Labels" and Label Threshold to 3%. This generates a MS/MS spectrum with m/z values labeled on the peaks.
- Export the MS/MS spectral data as a text file in the CSV format and reconstruct the spectrum using data processing software. Place m/z values on the peaks. The resulting MS/MS spectrum is shown in Figure 4.
NOTE: Some mass spectrometers are equipped with data processing software capable for generating the MS/MS spectrum. In this case, exporting the MS/MS spectral data is not necessary. - Assign the m/z values shown on the MS/MS spectrum to those shown in Table 1 to identify the corresponding B- and Y-ions. For example, the peak at m/z 580 is identified as the B4-ion and m/z 685 is identified as the Y5-ion. Label the peaks with corresponding B and Y symbols on the spectrum, as shown in Figure 4.
- Draw out the chemical structure of the acetylated peptoid by placing the N-terminus on the left side and the C-terminus on the right side, and draw a dashed line at each amide bond to generate a fragmentation scheme as shown in Figure 2a. Draw a proton with a dashed circle to indicate the charge carrier. Starting from the left side of the structure, place labels on the dashed lines to indicate the N-terminal fragments as B1, B2, to B8. Starting from the right side, place labels on the dashed lines to indicate the C-terminal fragments as Y1, Y2, to Y8.
Representative Results
The structure of a 9-mer peptoid with N-terminal acetylation, Ac-Nae-(Npe-Nme)4-NH2, is shown in Figure 1. The peptoid was synthesized manually in a fritted polypropylene reaction vessel via solid phase approach. Rink amide resin (0.047 mmol, 84 mg with loading 0.56 mmol/g) is used as the solid support to yield the peptoid with an amidated C-terminus. The peptoid chain is built by multiple cycles of monomer addition. Each monomer addition cycle involves two reactions steps, bromoacetylation and displacement. The bromoacetylation is achieved by adding 0.8 M BMA solution and 0.8 M DIC solution and the reaction takes 20 min. The displacement is achieved by adding 1.0 M amine solution to the acetylation product and the reaction takes 1 h. N-terminal acetylation was carried out by adding a cocktail solution containing 92 µL of acetic anhydride, 43.5 µL of DIPEA, and 2 mL of DMF. The peptoid is cleaved off from the resin by adding a cocktail solution containing 3.8 mL of TFA, 100 µL of TIPS, and 100 µL of HPLC-grade H2O, and the reaction takes 2 h. TFA is removed in the hood by blowing in a stream of nitrogen gas until about 1 mL viscous solution is left. The peptoid product precipitates in diethyl ether and is isolated by centrifugation, and this is followed by two iterations of lyophilization. The resulting peptoid is sufficiently pure for MS/MS analysis.
The predicted fragmentation scheme for the peptoid is shown in Figure 2a, where the proton in the dashed circle indicates the "mobile proton" that would induce peptoid fragmentation during the CID experiment. The peptoid ion fragments at the amide bonds along the peptoid backbone, for which the fragmentation sites are indicated by the dashed lines. The N-terminal fragments are labeled as B-type ions and the C-terminal fragments are labeled as Y-type ions. If the fragmentation occurs at all available amide bonds, a total of eight N-terminal fragments, B1 to B8, and a total of eight C-terminal fragments, Y1 to Y8, would form. Each fragment has a corresponding m/z value that is calculated by summing up the nominal masses of all elements in the fragment. As an example, the structures and the corresponding m/z values (nominal masses) of the B4-ion and the Y5-ion are shown in Figure 2b. The chemical formula for the B4 ion is C31H42N5O6+, and the nominal mass is calculated by the equation (31 x 12) + (42 x 1) + (5 x 14) + (6 x 16) = 580. Since B4 is a singly charged ion, the m/z value would be 580/1 = 580. In Figure 2b, the structure of the B4-ion is a simplified form (see discussion section for details). The calculated m/z values (nominal masses) of all fragment ions from B1-B8 and from Y1-Y8 are given in Table 1.
The mass analysis includes two processes. The first process is to perform the full scan mass spectrometry analysis of the peptoid sample. This result indicates whether the sample contains a measurable amount of the peptoid, and the relative purity of the sample. The full scan mass spectrum of the peptoid ion is shown in Figure 3, where the m/z values are rounded to the nearest whole number. The peak at m/z 1,265 corresponds to the protonated peptoid, and the peak at m/z 1,287 corresponds to the sodium ion adduct of the peptoid. The two peaks at m/z 633 and m/z 644 correspond to doubly protonated and mixed protonated-sodiated peptoids, respectively. These results suggest that the peptoid sample is sufficiently pure for carrying out MS/MS analysis.
The second process in mass analysis is to perform the MS/MS experiment on the protonated peptoid at m/z 1,265. This process includes isolating the precursor peptoid ion by the first quadrupole unit, fragmenting the peptoid ion in CID, and sorting the fragment ions according to their m/z values by the third quadrupole unit. The resulting spectrum is shown in Figure 4, where the m/z values are rounded to the nearest whole number. The peak at m/z 1,265 corresponds to the protonated peptoid. The other peaks at lower m/z values correspond to the fragment ions from the peptoid ion. The fragment ions are assigned as either the B-ion or the Y-ion by comparing their m/z values with those predicted (shown in Table 1) based on the peptoid fragmentation scheme (shown in Figure 2). Seven Y-ions (Y2 to Y8) and seven B-ions (B1 to B7) are formed with observable abundances. Notice that the abundance of the Y-ions is much higher than that of most B-ions.
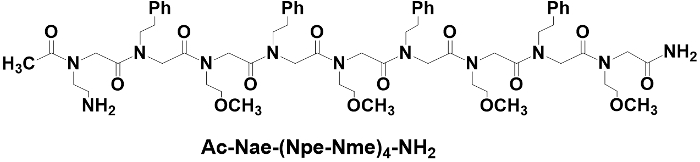
Figure 1: Chemical structure of the peptoid Ac-Nae-(Npe-Nme)4-NH2. The peptoid was synthesized manually via the solid phase approach. Please click here to view a larger version of this figure.
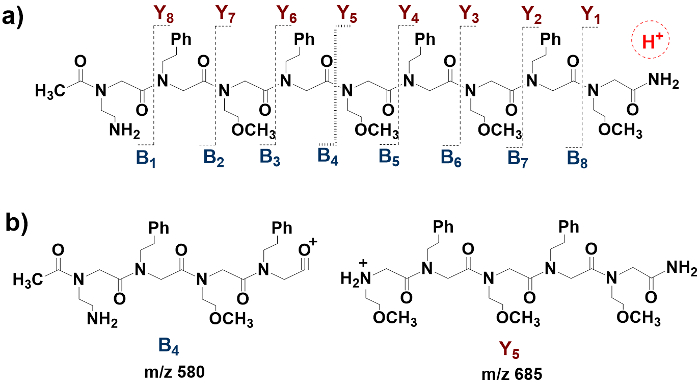
Figure 2: Fragmentation scheme for the protonated peptoid Ac-Nae-(Npe-Nme)4-NH2. The peptoid ion fragments at the amide bonds along the peptoid backbone to produce a series of N-terminal fragments called B-ions and a series of C-terminal fragments called Y-ions. a) Predicted fragmentation scheme for the protonated peptoid Ac-Nae-(Npe-Nme)4-NH2. The dashed lines indicate the fragmentation sites, the symbols B1 to B8 indicate the N-terminal fragments, and the symbols Y1 to Y8 indicate the C-terminal fragments; b) Sample fragmentation with schematic structures. The structures show the B4-ion and the Y5-ion with corresponding m/z values, respectively. The m/z values are calculated by summing up the nominal masses of the elements in the structures. Please click here to view a larger version of this figure.
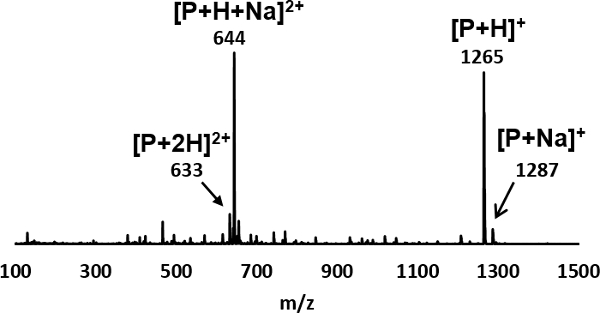
Figure 3: Full scan mass spectrum of the peptoid Ac-Nae-(Npe-Nme)4-NH2, where the m/z values are rounded to the nearest whole number. The peak at m/z 1,265 corresponds to the peptoid ion, [P+H]+, and the peak at m/z 1,287 corresponds to sodium ion adduct of the peptoid, [P+Na]+. The two peaks at m/z 633 and m/z 644 correspond to the doubly charged peptoid, [P+2H]2+ and [P+H+Na]2+, respectively. Please click here to view a larger version of this figure.
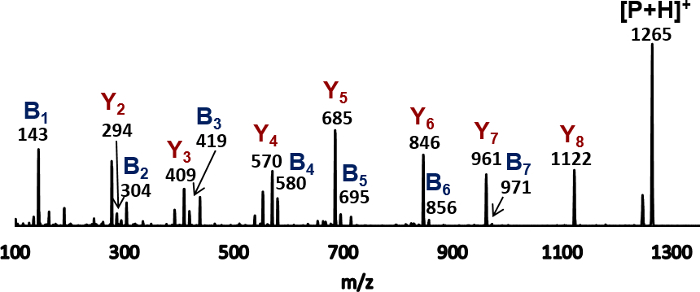
Figure 4: MS/MS spectrum of the protonated peptoid Ac-Nae-(Npe-Nme)4-NH2 labeled with assigned fragments. The peak at m/z 1,265 corresponds to the protonated peptoid, [P+H]+, and the peaks with lower m/z values correspond to the fragment ions. The B- and Y-ions are assigned by comparing their m/z values with those calculated based on the fragmentation scheme of the peptoid ion. Please click here to view a larger version of this figure.
| B-ion | m/z Value (Nominal Mass) | Y-ion | m/z Value (Nominal Mass) |
| B1 | 143 | Y1 | 133 |
| B2 | 304 | Y2 | 294 |
| B3 | 419 | Y3 | 409 |
| B4 | 580 | Y4 | 570 |
| B5 | 695 | Y5 | 685 |
| B6 | 856 | Y6 | 846 |
| B7 | 971 | Y7 | 961 |
| B8 | 1132 | Y8 | 1122 |
Table 1: Theoretical m/z values calculated based on the predicted fragmentation scheme of the protonated peptoid Ac-Nae-(Npe-Nme)4-NH2. The B-ion and the Y-ion indicate the corresponding fragment ions of the N-terminus and the C-terminus, respectively. Each m/z value (nominal mass) is calculated by summing up the nominal masses of the elements in that fragment ion.
Discussion
A nonamer peptoid, Ac-Nae-(Npe-Nme)4-NH2, has been synthesized using the protocol presented. The synthesis apparatus involves a syringe-like polypropylene solid-phase reaction vessel and a mechanical shaker. The reaction vessels are commercially available and low cost. A mechanical shaker is a common apparatus in chemistry laboratories. With the use of a syringe-like reaction vessel, solutions can be drawn into and pushed out of the vessel by manually moving the plunger. This technique allows the monomer addition and resin cleavage reactions to occur in one single reaction vessel and eliminates the step of transferring the resin-bound intermediate product into another vessel for cleavage. This technique also eliminates the need for a vacuum extraction device to remove solutions from the reaction vessel. Vacuum extraction is commonly used in manual peptoid synthesis that has been demonstrated by other researchers19,20. Thoroughly washing the resin between reaction steps is crucial to obtain a high purity product. One potential problem is the clogging of the frit in the reaction vessel after several cycles of monomer addition. A solution to this problem is to transfer the resin into a new reaction vessel and to continue the synthesis process. This synthesis technique works well for peptoids up to 10 – 12 residues. For longer peptoids, it becomes difficult to remove solutions from the reaction vessel by pushing the plunger. In this case, a vacuum extraction device can be utilized to remove solutions from the reaction vessel (the plunger should be replaced by a stopper). In the synthesis protocol, the crude product is purified by precipitating the peptoid in diethyl ether. Some shorter and highly polar peptoids may not form precipitate in diethyl ether. In this case, the peptoid can be isolated by dissolving the crude product in 10% acetic acid and washing the solution with diethyl ether multiple times, which is followed by lyophilization. Some highly hydrophobic peptoids may also not form precipitate in diethyl ether. In this case, evaporate the diethyl ether and use a reversed phase HPLC to purify the peptoid product.
In this study, the peptoid ion is generated by ESI and the MS/MS experiment is carried out in a triple-quadrupole mass spectrometer. Because of the ion isolation capability by the first quadrupole unit, the sample is directly infused into the ESI source, which eliminates the need for liquid chromatography (LC). Singly protonated peptoids can also be easily generated using the matrix-assisted laser desorption-ionization (MALDI) technique. The MS/MS experiments can be carried out using other types of mass spectrometers as well, such as an ion-trap spectrometer. Although the relative abundance of the fragment ions may appear different if the MS/MS spectra are recorded using different instruments, the qualitative spectral features should be similar. In general, the relative abundance of different fragment ions is highly sensitive to the instrument parameters. Changing the collision energy and the collision gas pressure can alter the relative abundance of the fragment ions significantly. For example, increasing the collision energy or increasing the collision gas pressure will promote fragmentation, and as a result, the abundance of the peptoid precursor ion will decrease and the abundance of the fragment ions will increase. This study focuses on singly charged peptoids. The length of the peptoids studied using this protocol is limited by the m/z range of the mass spectrometer. For a mass spectrometer with mass range up to m/z 2,000, the m/z values of the charged peptoids should be lower than the upper limit. If the peptoids have a molecular mass higher than the limit of the mass spectrometer, a doubly protonated peptoid can be used as the precursor ion. A doubly charged peptoid would have an m/z value which is approximately half of the singly charged one.
The peptoid presented in this work contains a basic residue with a primary amine as the side-chain group. The basic residue serves as a protonation site, which would enhance the ionization efficiency in the ESI source of the mass spectrometer. Less polar (more hydrophobic) peptoids have poor ionization efficiency in the ESI source. In this case, an atmospheric-pressure chemical ionization (APCI) source can be utilized to ionize the peptoids. Under the CID conditions, the peptoid ions mainly fragment at the amide bonds to produce a series of N-terminal fragments and a series of C-terminal fragments. If the charge carrier, the proton, resides on the N-terminal fragments, the B-ions form. Otherwise, the Y-ions form. Figure 2 shows a structure of the B4-ion. This is a simplified structure, which helps to calculate the m/z value. The actual B-ions are likely formed in a cyclic structure, such as a five-membered oxazolone ring23. Not all predicted fragment ions are formed in the mass spectrometer and observed in the mass spectrum. The efficiency of forming positively charged fragment ions is largely determined by the gas-phase basicity (or proton affinity) of the fragment. Highly basic fragments have a high chance to form positively charged ions and to be observed in the mass spectra. In general, observing at least 70% of the predicted fragment ions gives a reasonably high confidence for predicting the sequence of a peptoid. In designing peptoids for sequence analysis by mass spectrometry, it is important to place residues with polar side-chain groups, such as alkoxy groups, at different sites along the peptoid chain.
For most singly charged peptoids with N-terminal acetylation, the Y-ions show much higher abundance than the B-ions21,23. As shown in Figure 3, except that of the B1-ion, the intensity of the peaks corresponding to the B-ions is much lower than those of the Y-ions. For peptoids with free N-terminal amino groups, higher abundance of Y-ions over B-ions has been observed as well21. The favoring of Y-ions suggests that the charge-carrier proton prefers to reside on the C-terminal fragments, which is due to the higher proton affinity of the C-terminal fragments23. In addition to the formation of B- and Y-ions, secondary fragment ions associated with water loss or labile side-chain group loss are often observed in protonated peptoids. These secondary fragment ions often appear as a series of companion peaks next to the peaks of the primary fragment ions (especially Y-ions), and can be identified by subtracting the mass of the labile side-chain group from the mass of the corresponding primary fragment ions.
This protocol demonstrates how to manually synthesize an oligo-peptoid and analyze the monomer sequence of a peptoid using a tandem mass spectrometry method. This synthesis protocol can be easily adopted into a chemistry teaching laboratory to train new researchers in peptoid synthesis. This mass spectrometry protocol serves as an efficient tool, not only to confirm the identity of the peptoid, but also to characterize the structural features of the peptoid in relation to the observed fragmentation patterns. Future applications may involve developing a correlation map linking the peptoid structure and the fragmentation pattern through the synthesis and mass spectrometry analysis of a library of diverse peptoids.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Mr. Michael Connolly and Dr. Ronald Zuckermann (The Molecular Foundry, Lawrence Berkeley National Laboratory) for technique support in peptoid synthesis. We acknowledge the support from the National Science Foundation (CHE-1301505). All mass spectrometry experiments were conducted at the Chemistry Mass Spectrometry Facility at the University of the Pacific.
Materials
| ESI-triple quadrupole mass spectrometer, Varian 320L | Agilent Technologies Inc. | The mass spectrometer was acquired from Varian, Inc. | |
| Varian MS workstation, Version 6.9.2, a data acquisition and data review software | Varian Inc. | The software is a part of the Varian 320L package | |
| Burrell Scientific Wrist-action shaker, Model 75 DD | Fisher Scientific International Inc. | 14-400-126 | |
| Hermle Centrifuge, Model Z 206 A | Hermle Labortechnik GmbH | ||
| Solid phase reaction vessel, 10 mL | Torviq | SF-1000 | |
| Pressure caps for reaction vessels | Torviq | PC-SF | |
| Syringe filters, pore size 0.2 μm | Fisher Scientific Inc. | 03-391-3B | |
| Syringe filters, pore size 0.45 μm | Fisher Scientific Inc. | 03-391-3A | |
| Polypropylene centrifuge tuges, 50 mL | VWR International, LLC. | 490001-626 | |
| Polypropylene centrifuge tuges, 15 mL | VWR International, LLC. | 490001-620 | |
| ChemBioDraw, Ultra, Version 12.0 | CambridgeSoft Corporation | CambridgeSoft is now part of PerkinElmer Inc. | |
| Styrofoam cup, 12 Oz | Common Supermarket | ||
| Rink amide resin | Chem-Impex International, Inc. | 10619 | |
| Piperidine | Chem-Impex International, Inc. | 02351 | Highly toxic |
| N, N’-diisopropylcarbodiimide | Chem-Impex International, Inc. | 00110 | Highly toxic |
| Bromoacetic acid | Chem-Impex International, Inc. | 26843 | Highly toxic |
| 2-Phenylethylamine | VWR International, LLC. | EM8.07334.0250 | |
| 2-Methyoxyethylamine | Sigma-Aldrich Co. LLC. | 241067 | |
| N-Boc-ethylenediamine | VWR International, LLC. | AAAL19947-06 | |
| Acetic anhydride | Sigma-Aldrich Co. LLC. | 252845 | |
| N, N-dimethylformamide | VWR International, LLC. | BDH1117-4LG | Further distillation before use |
| N, N-diisopropylethylamine | Chem-Impex International, Inc. | 00141 | |
| Triisopropylsilane | Chem-Impex International, Inc. | 01966 | |
| Trifluoroacetic acid | Chem-Impex International, Inc. | 00289 | Highly toxic |
| Millipore MILLI-Q Academic Water Purification System | Millipore Corporation | ZMQP60001 | For generating HPLC grade water |
| HPLC-grade Water | Produced from Millipore MILLI-Q® Academic Water Purification System | ||
| Methanol | Pharmco-Aaper | 339USP/NF | HPLC grade |
| Acetonitrile | Fisher Scientific International, Inc. | A998-4 | HPLC grade |
| Diethyl ether | VWR International, LLC. | BDH1121-19L | Further distillation before use |
| Dichloromethane | VWR International, LLC. | BDH1113-19L | Further distillation before use |
| Nitrogen gas | Fresno Oxygen/Barnes Supply | NIT 50-C-F | Ultra high purity, 99.9995% |
| Argon gas | Fresno Oxygen/Barnes Supply | ARG 50-C-F | Ultra high purity, 99.9995% |
References
- Sun, J., Zuckermann, R. N. Peptoid Polymers: A Highly Designable Bioinspired Material. ACS Nano. 7 (6), 4715-4732 (2013).
- Fowler, S. A., Blackwell, H. E. Structure-function relationships in peptoids: Recent advances toward deciphering the structural requirements for biological function. Org. Biomol. Chem. 7 (8), 1508-1524 (2009).
- Chongsiriwatana, N. P., Patch, J. A., Czyzewski, A. M., Dohm, M. T., Ivankin, A., Gidalevitz, D., Zuckermann, R. N., Barron, A. E. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. U. S. A. 105 (8), 2794-2799 (2008).
- Kruijtzer, J. A., Nijenhuis, W. A., Wanders, N., Gispen, W. H., Liskamp, R. M., Adan, R. A. Peptoid-Peptide Hybrids as Potent Novel Melanocortin Receptor. J. Med. Chem. 48 (13), 4224-4230 (2005).
- Liu, B., Alluri, P. G., Yu, P., Kodadek, T. A Potent Transactivation Domain Mimic with Activity in Living Cells. J. Am. Chem. Soc. 127 (23), 8254-8255 (2005).
- Patch, J. A., Barron, A. E. Helical Peptoid Mimics of Magainin-2 Amide. J. Am. Chem. Soc. 125 (40), 12092-12093 (2003).
- Ham, H. O., Park, S. H., Kurutz, J. W., Szleifer, I. G., Messersmith, P. B. Antifouling Glycocalyx-Mimetic Peptoids. J. Am. Chem. Soc. 135 (35), 13015-13022 (2013).
- Olivier, G. K., Cho, A., Sanii, B., Connolly, M. D., Tran, H., Zuckermann, R. N. Antibody-Mimetic Peptoid Nanosheets for Molecular Recognition. ACS Nano. 7 (10), 9276-9286 (2013).
- Olsen, C. A., Ziegler, H. L., Nielsen, H. M., Frimodt-Moeller, N., Jaroszewski, J. W., Franzyk, H. Antimicrobial, Hemolytic, and Cytotoxic Activities of β-Peptoid-Peptide Hybrid Oligomers: Improved Properties Compared to Natural AMPs. ChemBioChem. 11 (10), 1356-1360 (2010).
- Lutz, J. -. F., Ouchi, M., Liu, D. R., Sawamoto, M. Sequence-Controlled Polymers. Science. 341 (6146), 628 (2013).
- Altuntas, E., Schubert, U. S. “Polymeromics”: Mass spectrometry based strategies in polymer science toward complete sequencing approaches: A review. Anal. Chim. Acta. 808, 56-69 (2014).
- Paulick, M. G., Hart, K. M., Brinner, K. M., Tjandra, M., Charych, D. H., Zuckermann, R. N. Cleavable Hydrophilic Linker for One-Bead-One-Compound Sequencing of Oligomer Libraries by Tandem Mass Spectrometry. J. Comb. Chem. 8 (3), 417-426 (2006).
- Thakkar, A., Cohen, A. S., Connolly, M. D., Zuckermann, R. N., Pei, D. High-Throughput Sequencing of Peptoids and Peptide-Peptoid Hybrids by Partial Edman Degradation and Mass Spectrometry. J. Comb. Chem. 11 (2), 294-302 (2009).
- Sarma, B. K., Kodadek, T. Submonomer Synthesis of A Hybrid Peptoid-Azapeptoid Library. ACS Comb Sci. 14 (10), 558-564 (2012).
- Li, X., Guo, L., Casiano-Maldonado, M., Zhang, D., Wesdemiotis, C. Top-Down Multidimensional Mass Spectrometry Methods for Synthetic Polymer Analysis. Macromolecules. 44 (12), 4555-4564 (2011).
- Figliozzi, G. M., Goldsmith, R., Ng, S. C., Banville, S. C., Zuckermann, R. N. Synthesis of N-substituted glycine peptoid libraries. Methods Enzymol. 267, 437-447 (1996).
- Zuckermann, R. N., Kerr, J. M., Kent, S. B. H., Moos, W. H. Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis. J. Am. Chem. Soc. 114 (26), 10646-10647 (1992).
- Utku, Y., Rohatgi, A., Yoo, B., Kirshenbaum, K., Zuckermann, R. N., Pohl, N. L. Rapid Multistep Synthesis of a Bioactive Peptidomimetic Oligomer for the Undergraduate Laboratory. J. Chem. Educ. 87 (6), 637-639 (2010).
- Tran, H., Gael, S. L., Connolly, M. D., Zuckermann, R. N. Solid-phase submonomer synthesis of peptoid Polymers and their self-assembly into highly-ordered nanosheets. J. Visualized Exp. (57), e3373 (2011).
- Bolt, H. L., Cobb, S. L., Denny, P. W. An Efficient Method for the Synthesis of Peptoids with Mixed Lysine-type/Arginine-type Monomers and Evaluation of Their Anti-leishmanial Activity. J Vis Exp. (117), (2016).
- Morishetti, K. K., Russell, S. C., Zhao, X., Robinson, D. B., Ren, J. Tandem mass spectrometry studies of protonated and alkali metalated peptoids: Enhanced sequence coverage by metal cation addition. Int. J. Mass Spectrom. 308 (1), 98-108 (2011).
- Bogdanov, B., Zhao, X., Robinson, D. B., Ren, J. Electron Capture Dissociation Studies of the Fragmentation Patterns of Doubly Protonated and Mixed Protonated-Sodiated Peptoids. J. Am. Soc. Mass Spectrom. 25 (7), 1202-1216 (2014).
- Ren, J., Tian, Y., Hossain, E., Connolly, M. D. Fragmentation Patterns and Mechanisms of Singly and Doubly Protonated Peptoids Studied by Collision Induced Dissociation. J. Am. Soc. Mass Spectrom. 27 (4), 646-661 (2016).

