Noninvasive, High-throughput Determination of Sleep Duration in Rodents
Summary
We describe a high-throughput method of measuring sleep by means of activity-based home-cage monitoring. This method offers advantages over traditional EEG-based methods. It is well validated for the determination of total sleep duration and can be a powerful tool to monitor sleep in rodent models of human disease.
Abstract
Traditionally, sleep is monitored by an electroencephalogram (EEG). EEG studies in rodents require surgical implantation of the electrodes followed by a long recovery period. To perform an EEG recording, the animal is connected to a receiver, creating an unnatural tether to the head-mount. EEG monitoring is time consuming, carries risk to the animal, and is not a completely natural setting for the measurement of sleep. Alternative methods to detect sleep, particularly in a high-throughput fashion, would greatly advance the field of sleep research. Here, we describe a validated method for detecting sleep via activity-based home-cage monitoring. Previous studies have shown that sleep assessed via this method has a high degree of agreement with sleep defined by traditional EEG-based measures. Whereas this method is validated for total sleep time, it is important to note that sleep bout duration should be assessed by an EEG which has better temporal resolution. The EEG can also differentiate rapid eye movement (REM) and non-REM sleep, giving more detail about the exact nature of sleep. Nevertheless, activity-based sleep determination can be used to analyze multiple days of undisturbed sleep and to assess sleep as a response to an acute event (like stress). Here, we show the power of this system to detect the response of mice to daily intraperitoneal injections.
Introduction
Sleep has important functions for the restoration of the body and brain following the daily burden of wakefulness1. It has been shown that sleep plays a role in memory retention and general brain plasticity1. The EEG is the gold standard to detect sleep2. In rodents, EEG monitoring requires surgical implantation of electrodes affixed to a head-mount, after which the animal needs a period of time to recover2. After the recovery, the animal is attached to the recording device and is given another period of habituation2. Because of these necessary periods of recovery and habituation, EEG is time consuming and laborious and cannot be reasonably performed on a large scale. Additionally, the surgical procedure of electrode implantation carries an inherent risk to the animal. Finally, the data analysis for scoring sleep in EEG studies is also very laborious. An alternative, non-invasive, high-throughput method of sleep monitoring would greatly aid rodent sleep research.
An activity-based home-cage monitoring system used to detect sleep addresses the limitations of EEG studies. The simple premise is that an inactive animal is likely a sleeping animal. It has been shown that 40 s of continuous inactivity (binned in 10 s epochs) is a reliable measure of sleep as measured with an EEG (shown to have 88-94% agreement)3. Home-cage monitoring systems can be used to study large groups of animals with minimal setup time. We have shown that it takes animals approximately one day to habituate to individual housing in the home-cage monitoring system4 in contrast to the weeks of recovery needed for EEG studies2. In addition, some setups can also detect physiological parameters such as core body temperature, heart rate, activity, and feeding. Temperature and heart rate are determined from the implantation of a small transmitter. These parameters can provide more information about the mouse and may be used in parallel with the sleep recording to further add to our understanding of sleep and how it is affected.
While it is a powerful tool, there are some limitations to the types of data that can be acquired from activity-based home-cage monitoring. EEG studies can differentiate between REM and non-REM sleep, which may be important for a deeper understanding of sleep architecture. Activity-based home-cage monitoring systems can only provide data for total sleep duration. In addition, although the output for activity-based home-cage monitoring gives information about sleep bout duration, we cannot accurately assess bout duration because of the inherent limitation of 40 s intervals3. Despite these limitations, home-cage monitoring of sleep duration provides an important biological measure that may influence many downstream factors including the animal's health and behavior5.
Activity-based home-cage monitoring has been used to detect sleep in many studies indicating its versatility. We cite a sample of these studies4,6,7,8,9,10,11,12. In addition to the method presented, there are other methods of detecting sleep via activity-based monitoring, each containing its own limitations13,14. Some of these studies examine long periods of uninterrupted sleep (72 h) while some examine sleep in blocks of 24 h. In this study, we present sleep analysis for each 24 h period after the response to daily intraperitoneal (IP) injections and to periodic cage changes in a mouse model of fragile X syndrome (Fmr1 KO mice). We chose Fmr1 KO mice because they have reduced sleep4 and are hypothesized to be hyper-reactive to sensory information15. Our data highlight the ability to detect changes in sleep patterns in response to a stressful event. This method is ideal for obtaining general information about sleep in large cohorts of mice. The method can be useful for understanding the effects of specific genetic alterations on sleep, the effects of pharmacological treatments, or responses to events, such as a stressor. In addition, the method provides a simple means of screening for a response before initiating more involved studies.
Protocol
All the procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guidelines on the Care and Use of Animals.
1. Setting up the Sleep Detection Units
- Purchase the desired number of units and software.
- Follow the instructions to set up the monitoring systems.
- Align a detector opposite to an emitter. Make sure the infrared beams are facing inwards and are aligned at the same height.
- Use the provided screws to position the detectors and emitters at the desired height on the metal stand. This height should be adjusted so that the bedding of the cage is below the level of the infrared beams, but the beam is at the proper height to detect the animal's activity. This creates an internal area of 27 cm × 32 cm (10.625 in × 12.75 in).
- If more screws are needed, purchase more at a hardware store (6-32 ¼ in Pan Head Screw).
- Connect each detector with each emitter. Connect the emitter to the provided hub linked to the receiver. Perform this for both the x and the y planes. Repeat this step for all setups.
- Connect the receiver to a computer with the provided USB hub.
2. Software Setup
- Install the analysis software and select the provided hardware configuration file as the default. The hardware configuration file should only be changed by the company.
- Click File | Open Experiment Configuration. Open the default experiment configuration.
- Click Experiment | Properties.
- Click the Scan tab. Change the Scan Rate to 10 s.
- Click the Activity tab. Change the activity sampling rate to 10 s.
- Click File | Save Experiment As to save these configuration settings for future experiments.
3. Animal Setup
- Singly house the mice in clean cages that are 31 cm (12.25 in) long and 16.5 cm (6.5 in) wide. To prevent the mouse from building up the bedding and obstructing the beams, use bedding at a depth of 3 mm. Do not provide additional nesting material for the mice.
- Provide the mice with access to food and water ad libitum by means of a wire feeder that rests in the top of the cage out of the way of the beams. If necessary, refill the food and change the water bottles when cages are changed every 3-5 days throughout the duration of the study.
- Align the mouse cage inside the infrared beam set up, ensuring that it rests in approximately the middle of the beams for full coverage.
- Ensure the light dark cycle of the room is set to mirror the light dark cycle of the normal housing conditions of the mice, or change as desired based on the experiment.
4. Drug Preparation and Injections
- Obtain sterile water and sterile cyclodextrin.
- For all injections, use a 1 mL syringe with a 25.5-30.5 G hypodermic needle. A new sterile needle and syringe should be used for each mouse.
- To reduce the time between injections, assemble all syringes in advance.
- For cyclodextrin injections: prepare cyclodextrin solution by adding 3 g of cyclodextrin to 10 mL of water to make a 30% cyclodextrin solution.
- Administer 0.3 mL of either saline or cyclodextrin via IP injection.
5. Software to Record Sleep
- Open the default hardware configuration file.
- Click File | Open Experiment Configuration. Open the desired or default experiment configuration.
- Click Experiment | Setup.
- Designate the location to save the data file as well as a location to save the backup file. The software requires that the data file be stored in the Program Files under the specific program folder.
NOTE: Given that some computers view the Program Files as sensitive, access to the data file may not be possible after the experiment. Therefore, it is recommended to save the backup file to another, non-sensitive area on the local computer. - Designate the animal ID to each sleep chamber and enter the weight of the animal if desired. If a chamber is not being used in the experiment, unchecking the box will deactivate that chamber. Once the identification information is entered, click Done.
- Designate the location to save the data file as well as a location to save the backup file. The software requires that the data file be stored in the Program Files under the specific program folder.
- Click File | Save Experiment As to save the current experiment configuration under the desired file name.
- Click Experiment | Run or F5 to start the recording. Wait for a 10 s epoch to make sure that all chambers are picking up activity. As the animals were just injected, it is highly likely that the animals will be moving enough to be detected by the infrared beams.
- If no activity is detected, try to move the mouse cage manually to ensure the beams pick up the movement. Continue troubleshooting or move to a working chamber if the activity is still not recorded.
NOTE: Run the experiment as soon as the injections are finished.
- If no activity is detected, try to move the mouse cage manually to ensure the beams pick up the movement. Continue troubleshooting or move to a working chamber if the activity is still not recorded.
- Cover the computer screens with an opaque cover to block the light. Cover the flashing lights on the activity meter.
- Run the experiment for 24 h.
NOTE: Because of the time necessary to perform the injections, it will not be a full 24 h. The injections should be performed at the same time daily. Sleep should not be recorded during injections. - The following day, at the time of injections, click Experiment | Stop to end the recording. Click File | Export | Generate Subject CSVs to collect the raw data for each mouse.
NOTE: It may take some time to export, so do this immediately and then proceed to injections while the computer is processing. - Click File | Export | Sleep to export the sleep file for each experiment by opening the raw .CDTA data file on any computer that has the software installed.
- Under Detection Parameters Activity Source, ensure that both the x-axis and y-axis boxes are checked.
- Under Sleep Threshold Epochs, ensure that 4 epochs are selected.
- Under Sleep Threshold Activity Threshold, ensure that 0 counts are selected.
- Under Light/Dark Cycle, check the appropriate time for the light/dark cycle.
- Under Analysis Window, select the desired time for analysis. In the case of the injection studies, leave the Day as default. Set the Start Time as ExpSTART. Set the Duration as 24:00 for 24 h. Analysis using the software can be done for a maximum of 72 h.
- Save the configuration to save time for exporting data.
- Click Update.
- Click Generate CSV File and save the file to the desired location.
6. Data Analysis
- For each recording session, open the sleep file. To assign the subject ID, open the individual CSV file for each chamber to determine the subject ID. Record the TOT sleep hh:mm:ss for both the light and the dark phases for all animals.
- Check the Individual subject CSV file for inconsistencies indicating the failure of recording. If high beam counts are detected in one plane, but no counts are observed on the other plane, this indicates beam failure.
NOTE: The "% sleeping" for the light and dark phases is calculated over the total recording period, which includes both light and dark phases. For 12 h light/dark cycles, multiply this number by 2 to get the "% sleeping" in the 12 h light or dark phase. Given that the injection studies did not go for a full 24 h, the calculated "% sleeping" is not accurate. To generate the correct "% sleeping", divide the "TOT sleep hh:mm:ss" by the "Experiment elapsed time: hh:mm:ss," multiply by 2, and then multiply by 100 to get the percent. If injections were performed in the light phase, then only the light phase needs to be adjusted in this manner.
- Check the Individual subject CSV file for inconsistencies indicating the failure of recording. If high beam counts are detected in one plane, but no counts are observed on the other plane, this indicates beam failure.
- Since different days and phases are being compared with each other, analyze the percent sleep duration for each phase and each day.
Representative Results
To determine the effect of daily injections on sleep and whether animals habituate to the injections, we performed daily IP injections for 14 consecutive days at 9:00 AM (light cycle began at 6:00 AM) and recorded sleep duration in 12 Fmr1 KO C57Bl/6J mice. We used a within subjects' design, injecting each animal with normal saline for 4 consecutive days (Days 1-4) and then 30% cyclodextrin for the following ten consecutive days (Days 5-14). Cyclodextrin was selected because it can be used to dissolve hydrophobic compounds for drug administration and we were interested in how vehicle injection may influence sleep in mice. Given the long duration of recording, we also changed cages two times per week throughout the study. Cage changes were performed at the same time as the injections on the days indicated. We report average percent sleep duration for the light and dark phase across the 14 days of injections and cage changes (Figure 1). Animals did not receive an additional habituation period to the sleep apparatus without injections. We found a significant phase x day interaction (F = 16.463) (p < 0.001), indicating that variation in sleep duration over the fourteen days differed between the light and dark phases. Post hoct-tests revealed that, in the light phase, sleep duration on Day 1 was different than that on almost all other days. This is consistent with the effects of the habituation to the sleep setup even without injections4. Also in the light phase, sleep duration on Days 6, 9, and 13 (when cages were changed following IP injections) was significantly different (p < 0.05) than sleep duration on neighboring days indicating that the cage change alters the sleep duration. While there is slight day-to-day variation in sleep durations (which is a normal occurrence), the significant decrease in sleep duration in the light phase when cages were changed suggests that cage changes affect sleep. There was no significant difference in sleep duration in the light phase on days of cyclodextrin administration without cage changes (i.e., Days 5, 7, 8, 10, 11, 12, and 14). These data indicate that the mice habituated to IP injections of cyclodextrin in a relatively short time. In the dark phase, sleep duration was different between Days 2 and 6 (the first cage change) suggesting potential compensation for the decrease in sleep duration in the light phase that occurred on Day 6. An alternate presentation of the sleep duration that breaks Days 4 to 6 into 2 h increments shows the immediate effect of injections and cage changes on sleep duration (Figure 2).
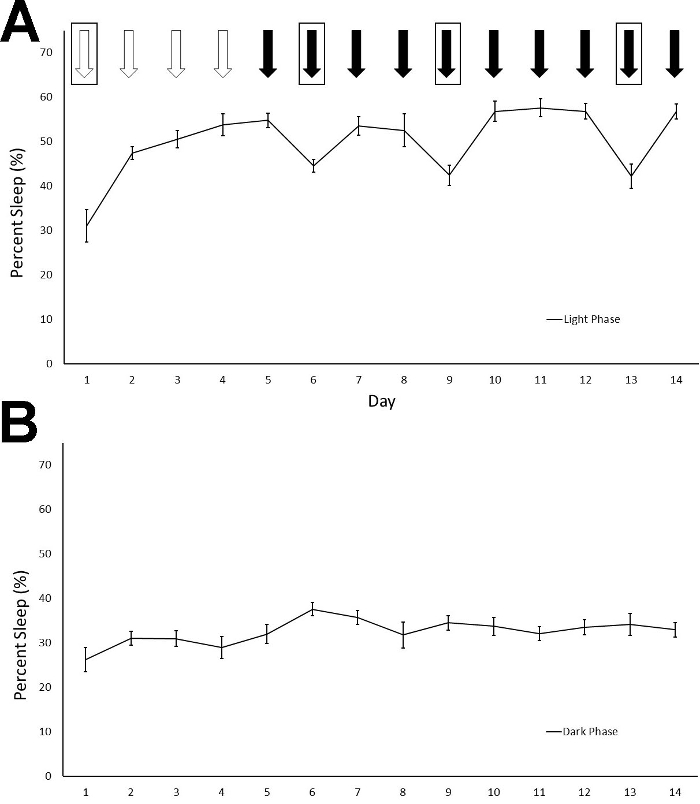
Figure 1: Percentage of sleep time in the light (A) and the dark phases (B) are shown across the fourteen-day recording period. Mice received daily IP injections of either saline (white arrows) or 30% cyclodextrin (black arrows) at 9 AM in the light phase. Boxes around the arrows indicate a cage change. The phase x day interaction was statistically significant (p < 0.001). Post hoc t-tests suggest that sleep duration differed in the light phase on Day 1 from other days, indicating the habituation to both the sleep setup and the IP injections. Sleep was reduced by cage changes on Days 6, 9, and 13 compared to other days (Day 5, 7, 8, 10 11, 12, and 14). Sleep duration following cyclodextrin injections was relatively stable across the days when cages were not changed indicating that the mice habituated to the IP cyclodextrin injections. Points represent means ± standard errors of the mean (SEM) in 12 mice. Please click here to view a larger version of this figure.
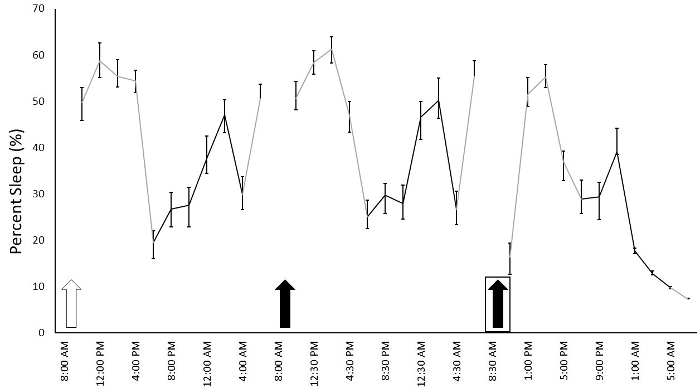
Figure 2: Percentage of sleep duration across 2 h frames beginning after saline injections on Day 4 and ending at the end of Day 6. Each data point is presented as the average sleep of the mice during the following a 2 h period. Mice received daily IP injections of either saline (white arrow) or 30% cyclodextrin (black arrows). Injections were given at 9 AM and sleep recording resumed after 1-1.5 h. Boxes around the arrows indicate a cage change. Cages were changed following injections at 9 AM and sleep recordings resumed at 11 AM. Gray lines indicate sleep that occurred in the light cycle while black lines indicate sleep that occurred in the dark cycle. Points represent means ± SEM in 12 mice. Please click here to view a larger version of this figure.
Discussion
Here, we present a noninvasive, high-throughput method for determination of sleep duration based on activity monitoring in the home-cage. This method of assessment of total sleep time has been validated against EEG studies3. Activity-based home-cage monitoring is simple, noninvasive, and applicable to population studies in large numbers of animals. It is limited in that it cannot give detailed information about sleep (such as sleep bout duration and sleep stages).
Pitfalls to this method of analysis are fairly easy to detect. It is important that none of the beams are obstructed during the study. This might occur by a build-up of bedding in an area of the cage. This can be minimized by limiting the height of the bedding to 3 mm and removing additional nesting material. This amount of bedding is sufficient to cover the cage bottom and low bedding volumes have not been found to affect stress levels in mice 17. Additional nesting material might also interfere with beam clearance, and therefore should not be provided. It is also important to ensure all beams are working properly during the setup procedure and to examine the cages before ending the study and extracting the data. If a beam is malfunctioning, an overestimation of sleep duration could occur. A beam malfunction or an obstruction can be detected during the data analysis phase by carefully looking at the CSV file. Instances of high counts on one axis without any counts on the other axis indicate that one of the sets of beams was malfunctioning or obstructed. This could be due to alignment issues (the detector not properly aligned with the emitter), connection issues, or equipment malfunction. Analysis of the sleep file only would miss this information, and sleep duration could be overestimated if it is not accounting for both directions of beams.
Another consideration of home-cage sleep monitoring is the need for single housing. Mice must be singly housed to ensure sleep recording is specific to each mouse being studied. Therefore, the duration of sleep assessment should be limited to prevent prolonged social isolation. Additionally, because animals need to be singly housed for home-cage monitoring, studying animals prior to weaning is not possible. Also, we have shown that it takes about 24 h to habituate to the single housing home-cage monitoring condition in mice at several different ages, but it may be necessary to test for habituation if different strains or transgenic mice that are being studied4. The results of the current study also suggest that cages should not be changed throughout the experiment to avoid the associated reduction in sleep.
This method has the power to study sleep in large numbers of animals with minimal labor and time. Therefore, it promises to be useful for behavioral phenotyping of different rodent lines and for assessing the effects of different manipulations (including pharmacological studies) on sleep.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the NIH Fellows Editorial Board for their editorial assistance. This research was funded by the Intramural Research Program of the NIMH (ZIA MH00889). RMS was also supported by a FRAXA Postdoctoral Fellowship.
Materials
| Comprehensive Lab Animal Monitoring System (CLAMS) | Columbus Instruments | Equipment and software to analyze sleep duration | |
| Captisol Research Grade | Captisol | RC-0C7-100 | Captisol for dissolving hydrophobic compounds |
| 30 G BD Needle 1/2 inch | BD | 305106 | Needle for injections |
| BD Disposable Syringes | Fisher | 14-823-30 | Syringes for injections |
| B6.129P2-Fmr1tm1Cgr/J | Jackson Labs | 3025 | Fmr1 KO mice |
| Super Mouse 750 Mouse Cage | Lab Products, Inc. | Homecages for the mice | |
| SANI-Chips Bedding | PJ Murphys | Bedding for the mice |
References
- Picchioni, D., Reith, R. M., Nadel, J. L., Smith, C. B. Sleep, plasticity and the pathophysiology of neurodevelopmental disorders: the potential roles of protein synthesis and other cellular processes. Brain sciences. 4, 150-201 (2014).
- Ingvar, M. C., Maeder, P., Sokoloff, L., Smith, C. B. The effects of aging on local rates of cerebral protein synthesis in rats. Monographs in neural sciences. 11, 47-50 (1984).
- Pack, A. I., et al. Novel method for high-throughput phenotyping of sleep in mice. Physiological genomics. 28, 232-238 (2007).
- Sare, R. M., et al. Deficient Sleep in Mouse Models of Fragile X Syndrome. Front Mol Neurosci. 10, (2017).
- Alvarez, G. G., Ayas, N. T. The impact of daily sleep duration on health: a review of the literature. Progress in cardiovascular nursing. 19, 56-59 (2004).
- Kincheski, G. C., et al. Chronic sleep restriction promotes brain inflammation and synapse loss, and potentiates memory impairment induced by amyloid-beta oligomers in mice. Brain, behavior, and immunity. 64, 140-151 (2017).
- Sare, R. M., Levine, M., Hildreth, C., Picchioni, D., Smith, C. B. Chronic sleep restriction during development can lead to long-lasting behavioral effects. Physiology & behavior. 155, 208-217 (2015).
- Moretti, P., Bouwknecht, J. A., Teague, R., Paylor, R., Zoghbi, H. Y. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Human molecular genetics. 14, 205-220 (2005).
- Guzman, M. S., et al. Mice with selective elimination of striatal acetylcholine release are lean, show altered energy homeostasis and changed sleep/wake cycle. Journal of neurochemistry. 124, 658-669 (2013).
- Vecsey, C. G., et al. Daily acclimation handling does not affect hippocampal long-term potentiation or cause chronic sleep deprivation in mice. Sleep. 36, 601-607 (2013).
- Bogdanik, L. P., Chapman, H. D., Miers, K. E., Serreze, D. V., Burgess, R. W. A MusD retrotransposon insertion in the mouse Slc6a5 gene causes alterations in neuromuscular junction maturation and behavioral phenotypes. PloS one. 7, e30217 (2012).
- Angelakos, C. C., et al. Hyperactivity and male-specific sleep deficits in the 16p11.2 deletion mouse model of autism. Autism research: official journal of the International Society for Autism Research. 10, 572-584 (2017).
- Fisher, S. P., et al. Rapid assessment of sleep-wake behavior in mice. Journal of biological rhythms. 27, 48-58 (2012).
- Mang, G. M., et al. Evaluation of a piezoelectric system as an alternative to electroencephalogram/ electromyogram recordings in mouse sleep studies. Sleep. 37, 1383-1392 (2014).
- Chen, L., Toth, M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. 신경과학. 103, 1043-1050 (2001).

