Levator Auris Longus Preparation for Examination of Mammalian Neuromuscular Transmission Under Voltage Clamp Conditions
Summary
The protocol described in this paper uses the mouse levator auris longus (LAL) muscle to record spontaneous and nerve-evoked postsynaptic potentials (current-clamp) and currents (voltage-clamp) at the neuromuscular junction. Use of this technique can provide key insights into mechanisms of synaptic transmission under normal and disease conditions.
Abstract
This protocol describes a technique to record synaptic transmission from the neuromuscular junction under current-clamp and voltage-clamp conditions. An ex vivo preparation of the levator auris longus (LAL) is used because it is a thin muscle that provides easy visualization of the neuromuscular junction for microelectrode impalement at the motor endplate. This method allows for the recording of spontaneous miniature endplate potentials and currents (mEPPs and mEPCs), nerve-evoked endplate potentials and currents (EPPs and EPCs), as well as the membrane properties of the motor endplate. Results obtained from this method include the quantal content (QC), number of vesicle release sites (n), probability of vesicle release (prel), synaptic facilitation and depression, as well as the muscle membrane time constant (τm) and input resistance. Application of this technique to mouse models of human disease can highlight key pathologies in disease states and help identify novel treatment strategies. By fully voltage-clamping a single synapse, this method provides one of the most detailed analyses of synaptic transmission currently available.
Introduction
Studying synaptic transmission at the neuromuscular junction provides insights into the dynamic relationship between the nervous and skeletal muscular systems and is an excellent model for examining synaptic physiology. The levator auris longus (LAL) is a thin muscle, allowing for the neuromuscular junctions to be easily visualized. Previous reports have described the convenience of using the LAL to examine synaptic drugs and toxins and have characterized the skeletal muscle fiber type characteristics of the LAL1,2. Numerous studies have used the LAL to examine neuromuscular physiology3,4,5,6,7,8. For electrophysiology, the ability to easily observe LAL neuromuscular junctions allows for the accurate placement of microelectrodes at the motor endplate and greatly reduces space clamp issues in recording synaptic transmission. Current-clamp recordings of the muscle membrane properties, such as the membrane time constant (τm) and input resistance (Rin) are readily obtained. Furthermore, these properties can be measured from the same muscle fibers used to record neuromuscular transmission, allowing for a direct comparison of synaptic function to the muscle membrane properties. Analysis of these data can provide key insights into the physical mechanisms of many neuromuscular diseases and states of altered activity.
A key aspect of the technique described here is the use of voltage-clamp for synaptic recordings, which are not subject to the non-linearities encountered in current-clamp and are independent of the muscle membrane properties. Advantages of using voltage-clamp as opposed to current-clamp to examine neuromuscular transmission were established by pioneering efforts in the 1950s9. Under current-clamp, EPPs that exceed 10-15 mV in amplitude are not a linear product of the mEPP amplitude9. For example, if the average mEPP is 1 mV, an EPP of 5 mV can be assumed to be the product of 5 mEPPs (QC of 5); whereas, an EPP of 40 mV will be the product of more than 40 mEPPs. This non-linearity at larger EPPs occurs because the driving force for the EPP, which is the difference between the membrane potential and equilibrium potential for the acetylcholine receptor (~-10 mV), substantially decreases during large EPPs. This issue is avoided in voltage-clamp experiments because the muscle membrane potential does not change during voltage-clamp experiments. A drawback is that voltage-clamp experiments are technically more difficult to complete than current-clamp recording. With this in mind, McLachlan and Martin developed a straightforward mathematical correction that accounts for non-linearities in current-clamp recordings of EPPs10. The corrections work well11,12,13, but importantly, assume that the muscle membrane properties have not been disrupted.
The muscle membrane properties are especially important to consider if studying conditions or disease states that disrupt the muscle. For example, skeletal muscle from the R6/2 transgenic model of Huntington's disease is hyperexcitable due to a progressive reduction in the resting chloride and potassium currents14,15. As a consequence, mEPPs and EPPs are amplified in the R6/2 skeletal muscle. Certainly, additional factors can alter mEPPs and EPPs. Work with a different model of Huntington's disease mice (R6/1) found changes in EPPs that seemed to be related to SNARE-proteins8. To assess the mechanisms causing altered neuromuscular transmission, it would be beneficial to eliminate the effects of altered muscle membrane properties by using a voltage-clamp. In a recent study, the R6/2 neuromuscular transmission was studied under both current- and voltage-clamp conditions using the technique described herein. The entirety of the motor endplates were voltage-clamped with less than 1% error by placing two microelectrodes within the length constant of the endplate16. It was shown that voltage-clamp and corrected current-clamp records yielded contrasting measurements of neuromuscular transmission in R6/2 muscle. This highlights that it may be difficult to correct EPPs for non-linearities if the muscle membrane properties have been altered and shows the benefits of obtaining voltage-clamp records that are independent of the muscle membrane properties. The protocol presented herein is ideal for examining conditions or disease states that affect synaptic transmission and the postsynaptic membrane properties.
Protocol
All animal procedures were performed in accordance with the Animal Care and Use Committee of Wright State University.
1. Mouse Euthanasia
- In a fume hood, place the mouse in an airtight glass anesthetizing chamber.
- Expose the mouse via inhalation to a lethal dose of isoflurane (saturating, or ~25%). Leave the mouse in the chamber until no breathing can be observed.
- Remove the mouse from the chamber and perform a cervical dislocation as a secondary method of euthanasia.
2. Removal of Hair from the Dorsal Surface of Head, Neck, and Back
- Use an electric shaver to remove the majority of the hair on the dorsal surface of the head, neck, and back of the mouse. Also, remove the hair around and on the left ear.
NOTE: Take care when shaving the skin around the ears, skin can get caught in the blades of the shaver and could damage underlying muscle tissue. - Apply hair removal cream with a cotton swab to the shaved area to remove the remaining hair. Wait approximately 1 min and rinse the hair removal cream with water using a squeeze wash bottle, then brush away any remaining cream or hair using a cotton swab and pat the skin dry.
3. Remove Skin to Expose the Levator Auris Longus Muscle
- Under a stereo dissecting microscope, make a small incision (just deep enough to penetrate the skin) on the back of the mouse at the level of the scapulae. Cut the skin using micro dissection scissors, following the path shown in Figure 1A-B.
- Using fine forceps (such as No. 5), pull up the skin along the cut near the scapulae. Using spring scissors, cut the connective tissue to separate the skin from the underlying muscle tissue while approaching the ear. Importantly, cut the connective tissue with the blades pointed into the skin to avoid accidental cutting of the exposed muscle tissue.
- Periodically perfuse the exposed muscle with a physiological saline solution, such as the following Na+ external buffer that consists of (in mM): 144 NaCl, 4 KCl, 1.2 CaCl2, 0.6 MgCl2, 5 glucose, 1 NaH2PO4, pH 7.4 with NaOH, and has an osmolality of 300 ± 5 mmol/kg. Once the connective tissue has been cut up to the left ear, cut the skin around the ear to remove the skin completely from the mouse and discard it.
NOTE: The LAL attaches to the base of the ear and can easily be damaged while removing the skin. It is good to leave around 1 mm of skin around the base of the ear to avoid cutting the LAL.
4. Removal of Levator Auris Longus Muscle and Surrounding Tissue
- Using spring scissors, start by cutting muscles that are inferior to the LAL, which connect to the spinal column between the scapulae. Start at the right scapula, just to the right of the midline, and cut toward the rostral end of the mouse, while remaining on the right side of the midline. Continue cutting all the way through to the end of the muscle tissue on the cranium.
NOTE: The LAL is the most superficial muscle connected to the medial side of the ear and the midline, as shown in Figure 2. For additional images, a book by E.C. Greene17 shows excellent, hand-drawn images of the LAL. - Use forceps to grab the cut tissue immediately to the right of the midline, then gently lift and begin cutting the tissue toward the left ear with the blades of the scissors pressed against the cranium to remove several layers of muscle that are inferior to the left LAL. Leave all of the layers attached temporarily to avoid accidentally cutting the LAL during removal.
- Cut with blades parallel to and pressed against the cranium to avoid cutting the nerve that innervates the LAL, which wraps around the ear canal, and enters the muscles on the medial side of the ear. Continue cutting through the ear canal, keeping as much of the nerve attached as possible.
NOTE: The nerve that supplies the LAL branches off of the facial nerve and should be preserved as it will be utilized later in the procedure. - Cut the fatty tissue that is just past the ear canal along the same path shown in Figure 1B on the ventrolateral portion of the ear. Also, cut along the left scapula as was done on the right side to remove the muscle completely from the mouse.
5. Levator Auris Longus Muscle Isolation
- Place the LAL, and surrounding tissue, into a container. Use a container in which the dissected tissue can be pinned to the bottom, such as a Petri dish with a silicone elastomer bottom. Bathe and frequently wash the tissue in a physiological saline solution.
- Cut off the pinna of the ear along the base of the ear, leaving the cartilaginous part of the ear attached to the LAL. Flip the muscle so that the inferior side is facing up (LAL on the bottom of the dish).
- Place a pin, such as an insect pin, through the ear canal to hold the prep in place. Then using smaller pins, pin the remaining tissue on the opposite side of the midline of the LAL. Using forceps, gently pull the skin on the lateral portion of the ear to stretch the muscle out and place a small pin through the skin. Repeat this step until the tissue is well-secured to the dish, as shown in Figure 3.
NOTE: Small dissection pins can be made by cutting the tips of acupuncture needles to the desired length. These small pins minimize damage to the tissue and can be used with water-immersion microscope objectives with long working distances. - Begin removing the muscles (shown in Figure 4), that are covering the LAL (auricularis superior, abductor auris longus, and the interscutularis), and those bound to the LAL via connective tissue and are especially tight near the midline, using No. 5 forceps and spring scissors.
- Using the forceps to pull up on the overlying muscle layer, cut the connective tissue with the blades oriented toward the muscle layer being pulled and take care to avoid cutting or nicking the LAL. Cut toward the midline and stop cutting about three quarters of the way to the midline. Then, cut parallel to the midline to remove each muscle layer. Keep removing muscle layers until only the LAL remains.
- Remove some of the remaining connective tissue that is covering the LAL, which aids in impalement of the electrodes. Gently use No. 5 forceps to pull the connective tissue away from the LAL and cut it away using spring scissors. Only remove tissue that can be done so easily without risk of damaging the LAL in the process. Remove any large nerves that innervate the inferior muscle layers remaining on the inferior surface of the LAL that may obstruct the visualization of the neuromuscular junctions.
6. Isolation of Nerve
- Identify the nerve that innervates the LAL using a nerve stimulator (for example, two platinum wires connected to a pulse generator); there will be several nerves running from the ear canal to the muscles. Touch the nerves with the nerve stimulator supplying some current (current will vary around 5 V). When the muscle contracts, the correct nerve has been identified.
- Carefully grab the tissue near the nerve and use spring scissors to separate the nerve from the tissue surrounding the ear. To minimize damage, keep most of the nerve embedded in some surrounding tissue, which will be used later to secure the nerve to the recording dish.
NOTE: The motor nerve that innervates the LAL is located on the medial side of the ear canal opening. - If using a bipolar stimulating electrode, remove the surrounding tissue only around a region of nerve, distal from the muscle. If using a suction electrode, remove surrounding tissue at the cut end of the nerve.
NOTE: This is a good stopping point. This is a good stopping point. If a break is needed the dissected LAL prep can be maintained in a physiological solution or constant perfusion for a couple of hours to ensure that harmful metabolites do not accumulate. Use a large volume of solution or constant perfusion to ensure that harmful metabolites do not accumulate. - Next, unpin and transfer the muscle to a perfusion chamber for electrophysiology experiments under an upright compound microscope.
NOTE: Use a perfusion chamber with a soft bottom to which the tissue can be securely pinned (Figure 5A). - Pin the muscle at the ends (ear and midline) and along the edge of the muscle. Position the nerve perpendicular to the muscle fibers and pin it to the bottom of the dish through some excess tissue that was left intact at the end of the nerve. Keep the tissue bathed in a physiological saline solution at all times.
NOTE: It is also helpful to have rounded, elevated material directly under the LAL muscle fibers. This extra support aids in electrode impalement and can be made from a small section of silicone elastomer cast in a 50 mL conical tube lying on its side. The rounded section of the elastomer can be secured to the bottom of the perfusion chamber using a small amount of fresh silicone. A diagram of the perfusion chamber is shown in Figure 5B-C. The muscle fibers can adhere very tightly to newly cast silicone elastomer (which is very hydrophobic) and may become damaged. It is helpful to coat or block newly cast silicone with protein, similar to the blocking step in an immunoblot. To block it, we have incubated newly cast silicone in a concentrated solution of bovine serum albumin overnight.
7. Electrophysiology Equipment Setup
- Prepare or thaw aliquots of a dye to aid in visualizing the neuromuscular junction, such as the mitochondrial dye 4-(4-diethylaminostyryl)-1-methylpyridinium18, which is light sensitive and should be stored away from light. Also, thaw electrode solutions. Vortex all aliquots to ensure that all solutes are in solution. Prepare a physiological saline solution containing 1 µM µ-Conotoxin GIIIB (µ-CTX) and 80 µM BTS (optional).
- Secure the perfusion chamber with the LAL to the microscope stage. Place the reference electrode into a cup filled with 3 M KCl, which is connected to the recording chamber via an agar bridge. Connect the reference electrode to the amplifier per manufacturer instructions.
- At this point, position the nerve-stimulating electrode on the nerve. Use either a suction electrode or a small bipolar electrode, as they work well for nerve stimulation.
- Position the stimulating electrode as far from the muscle as possible to minimize the number of stimulation artifacts in the recordings.
NOTE: The stimulating electrode should be controlled with a stimulus isolation unit that can control the voltage amplitude as necessary.
- Position the stimulating electrode as far from the muscle as possible to minimize the number of stimulation artifacts in the recordings.
- Slowly raise the voltage by turning the voltage knob on the stimulus isolation unit until contractions are observed. The point at which the contraction was first observed is the threshold. Once the threshold has been identified, set the voltage on the stimulus isolation unit at 1.5*threshold (or as defined by the experiment).
NOTE: Figure 6 shows the muscle prep set up under an upright microscope and the small bipolar electrode positioned on the nerve using a ball-joint manipulator. It is favorable to have the lowest voltage for stimulation possible while still being sufficiently above threshold. A lower stimulus voltage reduces the number of stimulation artifacts during recordings. Also, placing the stimulating electrode away from the muscle will decrease the size of the stimulation artifact. Sometimes the end of the nerve will be damaged from the dissection, so it may be necessary to experiment with where along the nerve the stimulator should be placed. - Remove the bathing saline solution and replace with 5 µM 4-Di-2-Asp. Expose the LAL prep to the 4-Di-2-Asp for 10 min to achieve adequate fluorescence for neuromuscular junction visualization (Figure 7).
- Prepare two electrode solutions, 3 M KCl and a K+ internal solution consisting of (in mM) 75 aspartate, 5 MgCl2, 15 Ca(OH)2, 5 ATP disodium, 5 phosphocreatine disodium, 5 glutathione, 20 MOPS, 30 EGTA, pH 7.2 with KOH.
NOTE: The solutions should be prepared in advance; the K+ internal solution can be stored in aliquots at -20 °C. - Fill the pulled glass capillary for the voltage-sensing electrode with 3 M KCl. Use the K+ internal solution (prepared in step 7.6) for the current-passing electrode; use a narrow nonmetallic needle (~34 gauge) attached to a 1 mL syringe to easily fill the glass capillaries. Gently tap the capillaries to remove air bubbles; ensure that each filled electrode has a resistance of 10-15 MΩ.
NOTE: Check and note the resistance of both electrodes using the data acquisition software. - After 10 min, exchange the 4-Di-Asp solution with the normal Ca2+ solution.
8. Identification of Neuromuscular Junction Using Fluorescence
- Using an upright microscope with standard bright-field and fluorescence illumination, look for a bright band of fluorescent green neuromuscular junctions running perpendicular to the muscle fibers along the prep as shown in Figure 7A. Use a low magnification water-immersion objective (~10X) to identify neuromuscular junctions
NOTE: The microscope should be equipped with a FITC cube (Ex: 480/40, Dichroic: 505LP, Em: 535/50), and an LED, laser, halogen lamp, or mercury lamp for fluorescence.To minimize photo bleaching, alternate between bright-field and fluorescence illumination.
NOTE: This band is usually more proximal to the base of the ear than to the midline. The band is the region where the neuromuscular junctions are most concentrated. - Switch to a higher magnification water-immersion objective (~40X) and identify a neuromuscular junction on the top layer of muscle to examine with electrophysiology (Figure 7B).
- Secure the filled pipettes into the pipette holders on the appropriate headstages, per manufacturer's instructions. Use micromanipulators to position the electrodes above the muscle membrane within 100 µm of the identified neuromuscular junction (Figure 7C). Position the electrodes using primarily bright-field microscopy; use fluorescence to confirm the location of the electrodes relative to the neuromuscular junction.
- First, use a low magnification (~10X) objective to locate the electrodes and then switch to a higher magnification (~40X) objective for final positioning of the electrodes. Do not impale the electrodes into the muscle at this point, first tune the electrodes.
9. Tuning and Impaling the Electrodes
- Once the electrodes are positioned above the desired fiber, zero and tune the voltage-sensing electrode by bridge balancing (or an analogous approach) and neutralizing the electrode capacitance per the manufacturer's instructions. Also, zero the current-passing electrode.
- Bring both electrodes down to the surface of the fiber. Slowly adjust the position of the electrodes on the way down to the fiber so that the electrodes remain oriented with respect to the neuromuscular junction, as described in steps 8.3 and 8.4.
- After both electrodes have contacted the surface of the muscle fiber, impale the blunt current-passing electrode first. Use techniques such as buzz (brief pulses of excess capacitance compensation), brief current pulses, or gently tapping the table to facilitate electrode impalement.
- Monitor the signal, using an oscilloscope or oscilloscope protocol in the data acquisition software, until a negative membrane potential is identified, which indicates that the electrode has been impaled.
- Depress the sharper voltage-sensing electrode onto the muscle to the level of the impaled current-passing electrode. Once both electrodes are in line with one another, impale the voltage-sensing electrode using the same techniques applied with the current-passing electrode.
- Upon successful impalement, using the controller for the amplifier, apply a constant negative holding current with the current passing electrode to compensate for any membrane damage due to electrode impalement. As the cell starts to slowly charge toward -80 mV, increase the holding current as needed to bring the cell to the desired resting potential (i.e., -80 to -85 mV).
NOTE: Avoid recording from fibers that require a holding current larger in absolute value than -25 nA in wild type muscle to minimize the chance of recording from unhealthy fibers. - Once the fiber is stable for a couple of minutes at the desired resting potential, proceed to recording muscle membrane properties or neuromuscular transmission.
10. Recording Postsynaptic Membrane Properties and Synaptic Transmission
- Start with current-clamp recordings by applying an equal number of positive and negative current steps to measure the corresponding membrane voltage responses, which will be used to determine motor endplate Rin and τm. To ensure passive properties, use small voltage responses that reach a steady state and have a linear voltage-current relationship16,19,20. Avoid recording from fibers with a damaged or unhealthy nerve, such fibers have a mEPP frequency of >3 Hz.
- Next, enter two-electrode voltage-clamp per the manufacturer's instructions. Voltage-clamp fibers at a membrane potential of -85 mV. Voltage-clamp settings should acheive signal-to-noise ratios that allow detection of mEPCs.
NOTE: Maintaining adequate voltage control can be an issue. We use two methods to minimize these issues. First, the fibers are impaled within 100 µm of the motor endplate as described previously, which is within the length constant of these fibers21. Second, we use the DC restore feature of the amplifier. This feature sets a very high voltage-clamp gain. Our previous work has described this method in detail and outlined a method for assessing the voltage-clamped endplate16. - Once in voltage-clamp, record synaptic currents (mEPCs and eEPCs) using various nerve-stimulation protocols, based on the requirements of the experiment.
- Record spontaneous mEPCs and EPCs using a low-frequency nerve stimulation protocol (≤0.5 Hz) which enables one to record EPCs without synaptic modulation (facilitation or modulation) to assess quantal content. To assess synaptic facilitation or depression, use a high-frequency nerve stimulation protocol (10 pulses at 50 Hz).
- Stimulate the nerve at the desired frequency via a stimulus isolation unit, which can be triggered using a pulse generator that is controlled using the data acquisition software.
- Once the voltage-clamp recordings are done, return the amplifier to a current-clamp setting, turn off all currents and remove the electrodes from the fiber. Check that the electrodes did not become clogged or drift in baseline voltage during the recording.
NOTE: Regarding drift, the electrode voltage should be 0 volts in the extracellular solution, as they were set before fiber impalement. For example, the recorded voltages would be uncertain if the voltage drifted during the experiment.
Representative Results
Figure 8 shows an example of the current pulses (Figure 8A) and the voltage responses (Figure 8B) from one LAL fiber under current-clamp from a 12-week-old wild type R6/2 mouse. The presence of mEPPs indicates that these records were taken from the motor endplate. The records were obtained in normal physiological saline solution. These current-clamp records can be analyzed to determine the Rin and τm of that fiber16,19,20.
A representative recording of an EPC and two mEPCs, obtained under voltage-clamp conditions, is shown in Figure 9A. The brief current deflection preceding the EPC is the artifact caused by nerve stimulation. The analysis of mEPCs and EPCs can be made quicker with event detection software. This tool allows the researcher to make a template that can then automatically detect the events within the recording. The events can be superimposed and exported into any data analysis software. Figure 9B shows the superimposed EPCs and mEPCs (inset) from a representative fiber.
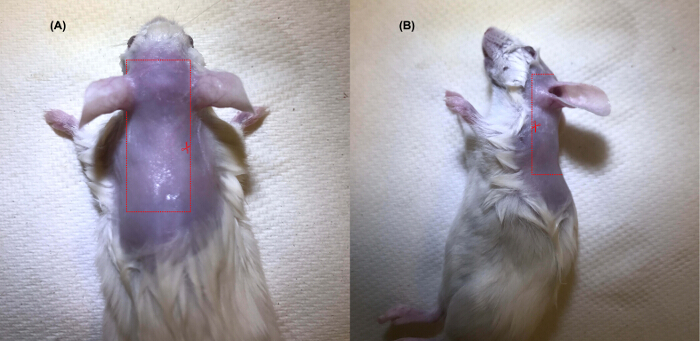
Figure 1: General location of the levator auris longus muscle
The LAL muscle is located on the dorsal surface of the head. The red dotted line highlights the path for cutting the skin for removal on the dorsal (A) and lateral (B) surface. The LAL attaches to the base of the ear, thus approximately 1 mm of skin around the base of the ear should remain intact to avoid cutting the LAL. Please click here to view a larger version of this figure.
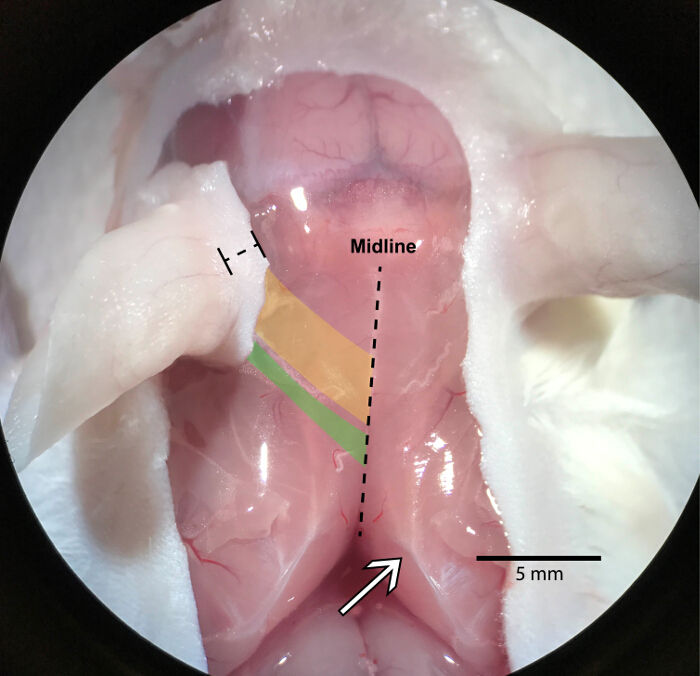
Figure 2: Exposed levator auris longus muscle
The LAL (yellow and green) is located just underneath the skin and is the most superficial muscle in the highlighted area. There are two portions of the muscle, the cranial (yellow) and caudal (green) regions. The cranial region emerges from the midline at the first four cervical vertebrae and runs toward the anterior part of the base of the auricle. For reference, the midline is a band of connective tissue that runs from the scapulae (white arrow) toward the nose. The right and left LAL muscle connect at the midline over the cranium. The cranial portion of the LAL is much wider than the caudal portion. The caudal portion attaches near the midline at the fourth and fifth cervical vertebrae and connects to the posterior part of the base of the auricle. During the dissection, keep a couple millimeters of skin around the cartilaginous ear to prevent cutting the LAL, which is marked in the figure by a bracketed dashed line. Please click here to view a larger version of this figure.
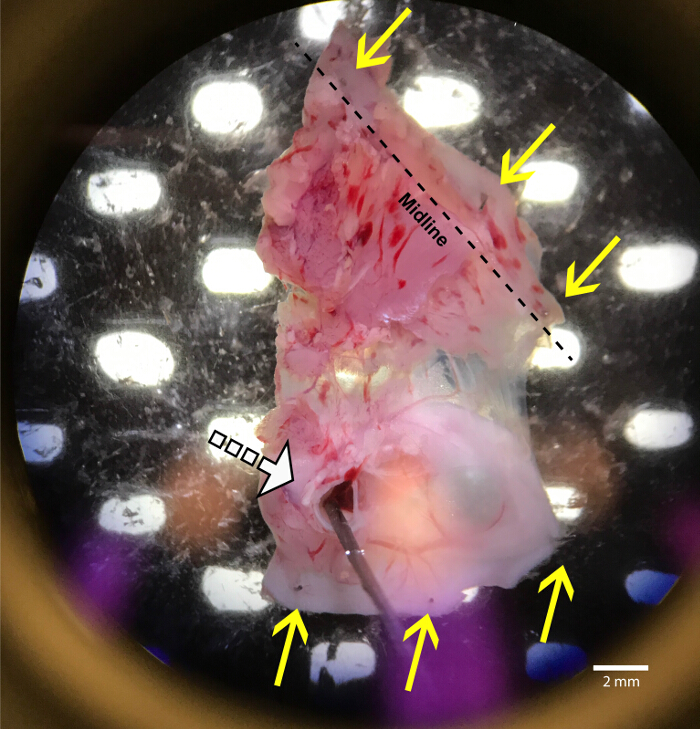
Figure 3: Crude dissection of the levator auris longus and surrounding muscle
Once removed from the animal, the tissue is pinned, dorsal side down (LAL on bottom), into a silicone elastomer lined dish using fine pins made as described in section 5.3. Once secured to the bottom of the dish, the overlying muscle layers can be removed. An insect pin through the ear canal is indicated with a white dashed arrow. The yellow arrows show ideal pin placement for removal of unwanted muscles. Please click here to view a larger version of this figure.
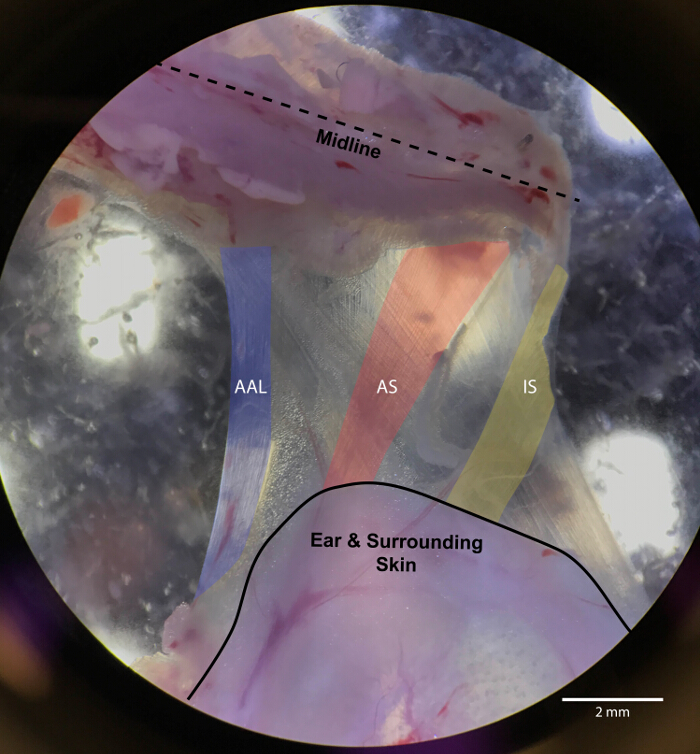
Figure 4: Muscle layer directly inferior to the levator auris longus
Highlighted in blue is the abductor auris longus (AAL), in red is the auricularis scupularis (AS), and in yellow is the interscutularis (IS). The LAL is the main, underlying muscle in this image. For reference, the ear and surrounding skin is outlined by a solid black line. Please click here to view a larger version of this figure.
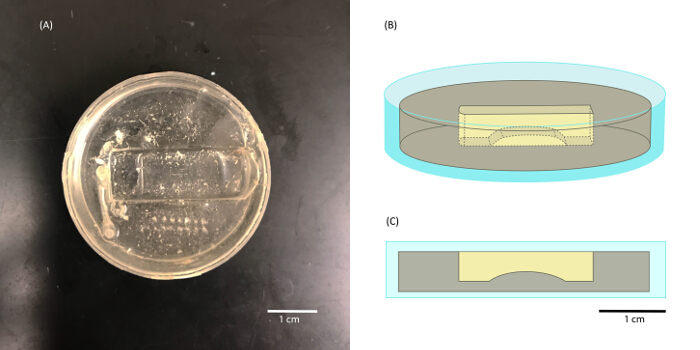
Figure 5: Silicone-elastomer-lined, custom perfusion chamber
Shown is the custom 35 mm perfusion dish used to secure the LAL for electrophysiological recordings (A). Silicone elastomer has been used to create a surface suitable for pining the tissue. In the center of the chamber is a rounded platform that is helpful when impaling the fibers. The platform supports the muscle fibers from underneath when applying a downward force with electrodes during the impalement process. This platform was shaped by allowing silicone elastomer to form to the curve of a 50 mL conical tube. A slice of this rounded silicone elastomer can then be glued to the bottom of the chamber with more silicone elastomer. An outlined representation of the dish as well as a side-on view are shown in B and C where the perfusion chamber can be seen more clearly. Please click here to view a larger version of this figure.

Figure 6: Electrophysiology experiment set-up
A small bipolar electrode is held in place with a magnetic ball-joint manipulator to stimulate the nerve of the LAL. The microscope stage can be made to easily hold a magnetic platform with the use of an adhesive magnetic material (as can be found at a craft or hardware store for making refrigerator magnets). Also shown are the headstages and electrodes positioned above an LAL sample. An important instrument is the water-immersion, beveled objective with a ceramic dipping cone, as shown. The beveled end allows for easier electrode placement and the ceramic material minimizes electrical noise. Please click here to view a larger version of this figure.
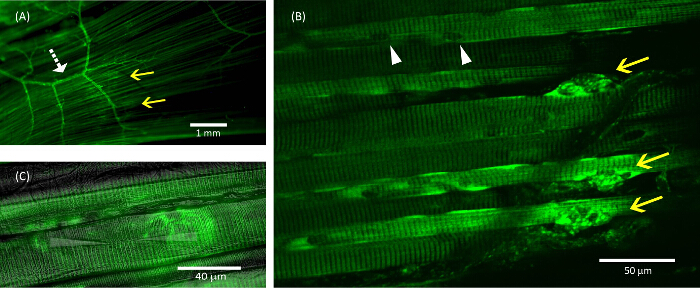
Figure 7: Neuromuscular junction identification
All images show the LAL stained with 5 µM 4-Di-2-Asp (Green) to allow for visualization of neuromuscular junctions. (A) A bright band of neuromuscular junctions can be seen (yellow arrows) when viewing the muscle through a 10X objective. Branching axons can also be seen as indicated by the white dashed arrow. (B) Small confocal stacks (5 x 1 µm) of three neuromuscular junctions (yellow arrows). Healthy muscle fibers have clear striations as well as multiple myonuclei that appear as dark spots along the sarcolemma of the fiber (white arrows). Often the axons innervating the neuromuscular junctions can be observed as well. (C) A fiber with a stained neuromuscular junction that is impaled with glass electrodes. The electrodes have been enhanced with a white highlight so that they can be seen more easily. Please click here to view a larger version of this figure.
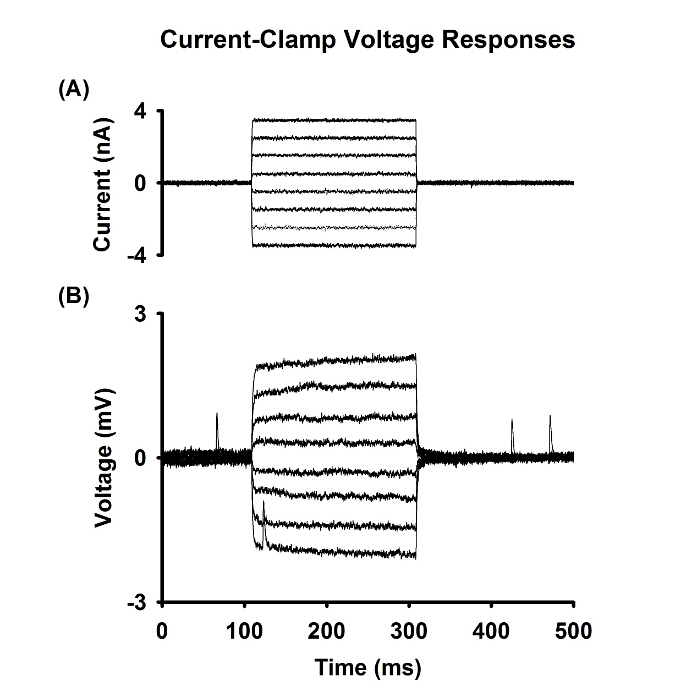
Figure 8: Current-clamp recording of membrane properties
Injected current pulses (A) and the resulting membrane potential responses (B) recorded from one fiber of the LAL bathed in physiologic saline solution. Please click here to view a larger version of this figure.
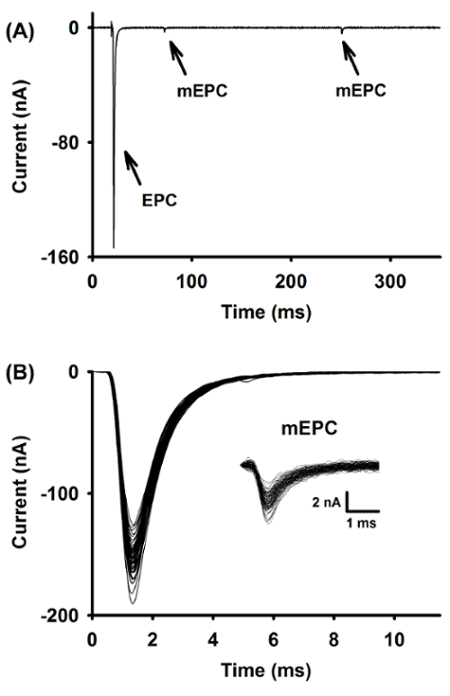
Figure 9: Two-electrode voltage-clamp recordings
A raw trace of an EPC and two mEPCs recorded in two-electrode voltage-clamp (A). Superimposed EPCs and mEPCs (inset) from a single fiber (B). Please click here to view a larger version of this figure.
Discussion
Described here is the preparation and use of the mouse LAL muscle for the measurement of neuromuscular transmission under current- or voltage-clamp conditions. There are several important points to consider for dissecting out the LAL. Cleaning excess connective tissue from the muscle aids in electrode impalement, as the electrodes can snag the connective tissue when positioning them for impalement. However, only remove connective tissue that can be taken away easily to limit the chances of damaging the muscle. The isolation of the nerve should be performed with care because it is very delicate. To avoid nerve damage, it is helpful to leave some of the surrounding tissue attached to the end of the nerve through which a pin can be placed to secure the nerve to the dish. Also, take care not to crush the nerve when positioning the nerve stimulator. Finally, the order in which the electrodes are impaled into the fiber is important. The blunt electrode is not as easy to impale and should be impaled first. If the sharp electrode were impaled first, the blunt electrode could push the muscle fiber down before it pierces the membrane, possibly causing the sharp electrode to come out of the fiber. This would make it necessary to impale the fiber a second time with the sharp electrode which would cause unnecessary membrane damage. It is also helpful that the amplifier being used can simultaneously measure the current and voltage of the current passing electrode so that a negative deflection in the membrane potential can be observed, indicating that the electrode has been impaled.
One feature of the LAL that can be beneficial is the ability to remove both muscles simultaneously. This can be great for performing electrophysiology and molecular biology experiments in the same animal. This can be accomplished by following essentially the same protocol described here with minor changes. Follow all steps under headings 1-3 doing everything described on the right side as well as the left. At step 4, it is best to begin cutting at the ventrolateral side of the right ear to remove the muscles. As described earlier, always keep the blades pressed against the skull to cut as deep as possible leaving inferior muscles attached to the LAL. Continue to cut towards the right ear past the midline, keeping the blade as deep as possible. At this point, the rest of the procedure is as described in this protocol, only perform the remaining steps on both muscles. Once the muscles have been cleaned, one LAL can be cut away and frozen for later molecular analysis and the other can be used for electrophysiology. It would be difficult to perform electrophysiology studies with both muscles of the same animal. To do so, the midline must be cut to separate the muscles and doing so will likely damage some muscle fibers.
The LAL has long been used to examine neuromuscular transmission because its thin nature allows for the easy identification of motor endplates and excellent ex vivo perfusion1,3,4,5,6,7,8. In addition to the use of 4-di-2-asp, other groups have used rhodamin-conjugated bungarotoxin at low concentrations22. We have used the LAL for electrophysiological examinations, purinergic signaling, and defects in Huntington's disease16,20. The LAL is also ideal for live-cell imaging studies. For example, several studies have used the LAL to measure synaptic vesicle release and uptake23,24. This can be done using dyes, such as FM 1-43.
Because the endplates can be easily observed with a fluorescent stain, the electrodes can be placed in close proximity to the motor endplate within the length constant of the muscle fiber. Electrode placement at the endplate and the use of a high compliance two-electrode voltage-clamp system enables investigators to fully control the endplate potential with less than 1% error16. This can be important because the commonly used corrections for non-linear summation of synaptic potentials recorded under current-clamp conditions10 do not account for changes in the postsynaptic membrane caused by experimental conditions and/or disease state. For example, reduced resting endplate conductances will amplify postsynaptic potentials19, even those corrected for non-linear summation. In contrast, voltage-clamped postsynaptic currents are not subjected to non-linear summation errors and are independent of the postsynaptic membrane properties. Demonstrating this, we have recently shown contrasting results from endplate potentials compared to currents recorded from hyper-excitable Huntington's disease skeletal muscle16.
A final key advantage of using the LAL for studying neuromuscular transmission is that recordings are from a single synapse. Neuromuscular transmission recorded from a single, fully voltage-clamped endplate provides some of the most detailed and accurate data on synaptic transmission currently available, which is ideal for modeling and unraveling the complexity of synaptic physiology.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Mark M. Rich and Daniel Miranda for editorial comments, Ahmad Khedraki for helping establish this technique, and Wright State University for financial support (startup fund to A.A.V.).
Materials
| Olympus Compound Microscope | Olympus | BX51WI | |
| 10x Objective | Olympus | UMPLFLN10XW | |
| 40x Objective | Olympus | LUMPLFLN40XW | |
| Borosilicate Glass | Sutter Instruments | BF150-86-7.5 | |
| CCD Camera | Santa Barbara Instruments Group | ST-7XMEI | |
| Axoclamp 900A Amplifier | Molecular Devices | 2500‐0179 | |
| Mater-9 Pulse Generator | AMPI | ||
| Iso-flex Stimulus Isolator | AMPI | ||
| pCLAMP 10 Data Acquisition and Analysis Software | Molecular Devices | 1-2500-0180 | |
| Concentric Bipolar Electrode | FHC | CBDSH75 | |
| Ball-joint Manipulator | Narishige | ||
| Non-metalic Syringes 34 Gauge | World Precision Instruments | MF34G-5 | |
| Nikon Stereomicroscope | Nikon | SMZ800N | |
| No. 5 Forceps | Fine Science Tools | ||
| Spring Scissors | Fine Science Tools | 15006-09 | |
| No. 2 Forceps | Roboz | RS-5Q41 | |
| Microdissecting Scissors | Roboz | RS-5912SC | |
| Sylgard 184 Silicone Elastomer Kit | Dow Corning | 2404019862 | |
| Hair Removal Cream | Nair | ||
| Grass SD9 Stimulator | Grass Medical | ||
| Model P-1000 Micropipette Puller | Sutter Instruments | P-1000 | |
| Axon Digidata 1550 Low-noise Data Acuisition System | Molecular Devices | ||
| Low Pass Bessell Filter | Warner Instrument Corp. | LPF-8 | |
| Left-handed Micromanipulator | Siskiyou Corp. | MX1641/45DL | |
| Right-handed Micromanipulator | Siskiyou Corp. | MX1641/45DR | |
| Single Motion Controler | Siskiyou Corp. | MC100e | |
| Crossed Roller Micromanipulator | Siskiyou Corp. | MX1641R | This was added to the Z-axis of the Left and Right-handed micromanipulators to allow the z axis to be motorized. This custom set-up is cheaper and less bulky than buying a 4-axis motorized micromanipulator. It also allows us to control both micromanipulators with one controller |
| All chemicals were orded from Fisher except, | |||
| BTS | Toronto Research Chemicals | B315190 | |
| CTX | Alomone Labs | C-270 | |
| 4-Di-2-Asp | Molecular Probes | Molecular probes is no longer a company. Now ordered through Fisher | |
References
- Angaut-Petit, D., Molgo, J., Connold, A. L., Faille, L. The levator auris longus muscle of the mouse: a convenient preparation for studies of short- and long-term presynaptic effects of drugs or toxins. Neurosci Lett. 82 (1), 83-88 (1987).
- Erzen, I., Cvetko, E., Obreza, S., Angaut-Petit, D. Fiber types in the mouse levator auris longus muscle: a convenient preparation to study muscle and nerve plasticity. J Neurosci Res. 59 (5), 692-697 (2000).
- Bertone, N. I., et al. Carbonic anhydrase inhibitor acetazolamide shifts synaptic vesicle recycling to a fast mode at the mouse neuromuscular junction. Synapse. , (2017).
- Garcia-Chacon, L. E., Nguyen, K. T., David, G., Barrett, E. F. Extrusion of Ca2+ from mouse motor terminal mitochondria via a Na+-Ca2+ exchanger increases post-tetanic evoked release. J Physiol. 574 (Pt 3), 663-675 (2006).
- Murray, L. M., et al. Selective vulnerability of motor neurons and dissociation of pre- and post-synaptic pathology at the neuromuscular junction in mouse models of spinal muscular atrophy. Hum Mol Genet. 17 (7), 949-962 (2008).
- Nadal, L., et al. Presynaptic muscarinic acetylcholine autoreceptors (M1, M2 and M4 subtypes), adenosine receptors (A1 and A2A) and tropomyosin-related kinase B receptor (TrkB) modulate the developmental synapse elimination process at the neuromuscular junction. Mol Brain. 9 (1), 67 (2016).
- Rousse, I., St-Amour, A., Darabid, H., Robitaille, R. Synapse-glia interactions are governed by synaptic and intrinsic glial properties. 신경과학. 167 (3), 621-632 (2010).
- Rozas, J. L., Gomez-Sanchez, L., Tomas-Zapico, C., Lucas, J. J., Fernandez-Chacon, R. Increased neurotransmitter release at the neuromuscular junction in a mouse model of polyglutamine disease. J Neurosci. 31 (3), 1106-1113 (2011).
- Takeuchi, A., Takeuchi, N. Further analysis of relationship between end-plate potential and end-plate current. J Neurophysiol. 23, 397-402 (1960).
- McLachlan, E. M., Martin, A. R. Non linear summation of end plate potentials in the frog and mouse. The Journal of Physiology. 311 (1), 307-324 (1981).
- Obis, T., et al. The novel protein kinase C epsilon isoform modulates acetylcholine release in the rat neuromuscular junction. Mol Brain. 8 (1), 80 (2015).
- Silveira, P. E., et al. Ryanodine and inositol triphosphate receptors modulate facilitation and tetanic depression at the frog neuromuscular junction. Muscle Nerve. 52 (4), 623-630 (2015).
- Wood, S. J., Slater, C. R. Safety factor at the neuromuscular junction. Prog Neurobiol. 64 (4), 393-429 (2001).
- Miranda, D. R., et al. Progressive Cl- channel defects reveal disrupted skeletal muscle maturation in R6/2 Huntington’s mice. J Gen Physiol. 149 (1), 55-74 (2017).
- Waters, C. W., Varuzhanyan, G., Talmadge, R. J., Voss, A. A. Huntington disease skeletal muscle is hyperexcitable owing to chloride and potassium channel dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 110 (22), 9160-9165 (2013).
- Khedraki, A., et al. Depressed Synaptic Transmission and Reduced Vesicle Release Sites in Huntington’s Disease Neuromuscular Junctions. Journal of Neuroscience. 37 (34), 8077-8091 (2017).
- Greene, E. C. . The anatomy of the rat. , (1955).
- Magrassi, L., Purves, D., Lichtman, J. W. Fluorescent probes that stain living nerve terminals. J Neurosci. 7 (4), 1207-1214 (1987).
- Jack, J. J. B., Noble, D., Tsien, R. W. . Electric current flow in excitable cells. , (1983).
- Voss, A. A. Extracellular ATP inhibits chloride channels in mature mammalian skeletal muscle by activating P2Y(1) receptors. Journal of Physiology-London. 587 (23), 5739-5752 (2009).
- Albuquerque, E. X., McIsaac, R. J. Fast and slow mammalian muscles after denervation. Experimental Neurology. 26 (1), 183-202 (1970).
- Santafe, M. M., Urbano, F. J., Lanuza, M. A., Uchitel, O. D. Multiple types of calcium channels mediate transmitter release during functional recovery of botulinum toxin type A-poisoned mouse motor nerve terminals. 신경과학. 95 (1), 227-234 (2000).
- Gaffield, M. A., Betz, W. J. Synaptic vesicle mobility in mouse motor nerve terminals with and without synapsin. J Neurosci. 27 (50), 13691-13700 (2007).
- Zhang, Z. S., Nguyen, K. T., Barrett, E. F., David, G. Vesicular ATPase Inserted into the Plasma Membrane of Motor Terminals by Exocytosis Alkalinizes Cytosolic pH and Facilitates Endocytosis. Neuron. 68 (6), 1097-1108 (2010).

