Preparation of Graphene Liquid Cells for the Observation of Lithium-ion Battery Material
Summary
Here, we present a protocol for the fabrication and preparation of a graphene liquid cell for in situ transmission electron microscopy observation, along with a synthesis of electrode materials and electrochemical battery cell tests.
Abstract
In this work, we introduce the preparation of graphene liquid cells (GLCs), encapsulating both electrode materials and organic liquid electrolytes between two graphene sheets, and the facile synthesis of one-dimensional nanostructures using electrospinning. The GLC enables in situ transmission electron microscopy (TEM) for the lithiation dynamics of electrode materials. The in situ GLC-TEM using an electron beam for both imaging and lithiation can utilize not only realistic battery electrolytes, but also the high-resolution imaging of various morphological, phase, and interfacial transitions.
Introduction
Recently, the consumption of energy has constantly increased, as well as the importance of high-performance energy storage devices. To meet such a demand, the development of lithium-ion batteries that have a high energy density, durability, and safety is necessary1,2. In order to develop batteries with superior properties, a fundamental understanding of energy storage mechanisms during battery operation is essential3,4,5.
In situ transmission electron microscopy (TEM) provides rich insights as it can show both structural and chemical information during the operation of batteries3. Among many in situ TEM techniques, GLCs have been used for the observation of the lithiation dynamics of nanomaterials6,7,8,9,10,11,12. GLCs consist of a liquid pocket sealed by two graphene membranes, which provide an actual electrode/electrolyte interface by preventing the evaporation of the liquid inside the high vacuum in a TEM column6,7. The advantages of GLCs are that they allow a superior spatial resolution and high imaging contrast because they employ electron transparent monatomic-thick graphene as liquid sealing membrane13,14,15,16. Also, conventional TEM can be applicable to observe the battery reactions, without using expensive in situ TEM holders.
In this text, we introduce how the lithiation reaction can be observed with GLCs. Specifically, electron beam irradiation produces solvated electrons inside the liquid electrolyte, and they initiate lithiation by separating Li ions from solvent molecules.
GLCs also serve as the most optimal platform to allow the direct observation of nanomaterials with various morphologies, including nanoparticles6,9, nanotubes7,10,11, and even multidimensional materials12. Together with the ex situ TEM analysis of electrode materials after the actual electrochemical cell testing, it is possible that the GLC system presented here can be used to investigate the fundamental reaction mechanism.
With such advantages of GLCs and ex situ experiments, we introduce here detailed experiment methods for researchers who are willing to carry out similar GLC experiments. The protocols cover 1) the synthesis of tin (IV) oxide (SnO2) nanotubes as the typical one-dimensional nanostructured electrode materials, 2) the electrochemical battery cell test, 3) the preparation of GLC, and 4) the performance of a real-time TEM observation.
Protocol
1. Synthesis of SnO2 Nanotubes by Electrospinning and Subsequent Heat Treatment17
- Prepare an electrospinning solution.
- Dissolve 0.25 g of tin chloride dihydrate in a solvent mixture of 1.25 g of ethanol and 1.25 g of dimethylformamide (DMF) at room temperature (RT, 25 °C).
- After stirring for 2 h, add 0.35 g of polyvinylpyrrolidone (PVP) to the electrospinning solution and stir the mixture for another 6 h.
- Perform an electrospinning of the Sn precursor/PVP composite nanofibers.
- When the electrospinning solution is well prepared, wash the rectangular, flexible stainless steel (30 x 20.5 cm) with deionized (DI) water and ethanol 2x – 5x and air-dry it at 60 °C for 10 min.
- Transfer the electrospinning solution to a 10 mL syringe by blocking one side of the syringe and letting the electrospinning solution flow into the syringe.
- Connect a 25 G needle to the syringe.
NOTE: Other types of needles, such as 23 G and 17 G needles, can also be used. In this protocol, the 25 G needle is used as the standard needle. - Fix the dried flexible stainless steel into the drummer with tape.
- Open the electrospinning controller software and enter the electrospinning parameters (flow rate: 5 – 20 µL·min-1, total amount of the solution: 3 – 15 mL) for the proper functioning of the electrospinning device.
NOTE: Here, the optimal flow rate is 10 µL·min-1 and the total amount of solution is 5 mL. - Fix the syringe with the 25 G needle into the electrospinning device and, then, fix it with tape.
- Before electrospinning, press the syringe towards the collector until the electrospinning solution flows well through the 25 G needle. Then, connect the tip of the needle to the double-ended crocodile clips, which are also connected to the collector.
- Roll the roller (100 rpm) and initiate the electrospinning program software.
- Modulate the applied voltage by using a voltage bias (Figure 1a) between 10 – 25 kV to allow the electrojetting of the Taylor cones to operate the electrospinning process.
NOTE: Here, the optimal applied voltage is 16 kV.
- Perform the calcination of the Sn precursor/PVP composite nanofibers.
- When the electrospinning process is finished, scrape the as-spun nanofibers on the flexible stainless steel with a razor and transfer them into an alumina box.
- Insert the alumina box into the box furnace and set the heat treatment conditions for the box furnace: 600 °C or 700 °C for 1 h, with a ramping rate of 10 °C·min-1.
- After calcination, cool down the furnace to 50 °C and, then, transfer the calcined samples (i.e., SnO2 nanotubes) to the vial glass (Figure 1b).
2. Electrochemical Battery Cell Test
- Prepare the electrode.
NOTE: The electrode slurry is composed of 10 wt% binder, 10 wt% carbon black, and 80 wt% of active materials (in this case, SnO2 nanotubes). The amount of slurry and the composition of each ingredient in the slurry can be adjusted.- Cut the copper (Cu) foil into 10 cm width x 30 cm length and, using ethanol, fix it on a rectangular glass substrate (25 x 15 x 0.5 cm).
- Mix 0.02 g of carbon black, 0.027 g of polyacrylic acid (35 wt%), and 0.166 g of carboxymethyl cellulose (6 wt%) in a crucible. Add three to six drops of deionized (DI) water into the crucible to allow for a homogeneous mixing.
- Add 0.16 g of SnO2 nanotubes into the crucible. Then, add three to eight drops of DI water into the crucible to allow for a homogeneous mixing.
- Ground all the ingredients in the crucible for 30 min to ensure that the slurry is sufficiently viscous to be cast well on the Cu foil.
- When the electrode slurry is prepared well, place the slurry on the top side of the Cu foil on the glass substrate, and cast it evenly using a casting roller.
NOTE: Usually, the thickness of the slurry is 60 µm but can be more or less. - Air-dry the slurry-cast Cu foil at 60 °C for 10 min and seal it inside the plastic bag prior to the battery cell assembly.
NOTE: The slurry-cast Cu foil can be seen in Figure 1c.
- Assemble the battery cells.
- Heat the convection oven to 150 °C. Then, place the slurry-cast Cu foil into the oven.
- Fill the convection oven with vacuum by rotary pumps to dry the residual solvent in the slurry while avoiding the oxidation of the Cu foil.
- After heating the slurry-cast Cu foil at 150 °C for 2 h, refill the convection oven with air by closing the vacuum line and opening the vent line in the rotary pump to open the chamber.
- Take the slurry-cast Cu foil out of the chamber and punch it with a circle puncher (punch diameter: 14 mm). Weigh the punched slurry-cast Cu foil and place it into the bottom battery cell.
- Use half a cell for the assembly of the battery cells. After placing the slurry-cast Cu foil into the bottom of the battery cell, transfer the samples to the antichamber of the glove box.
- Vacuum the antichamber for 30 min and, then, transfer the samples into the inside glove box.
- Assemble the battery cells in the following order: bottom battery cell, slurry-cast Cu foil, separator, gasket, spring, spacer, and top battery cell. Drop the electrolyte after the separator is put into the battery cell.
- Compress the battery cell into a complete battery cell by a compactor.Then, move the battery cells for electrochemical tests into the antichamber in the glove box and take the battery cells out of the glove box.
- Measure the open circuit voltage (OCV) by a digital multimeter and age the battery cell at RT for 1 – 2 d.
- Test the electrochemical battery cell.
- Calculate the weight of the active materials by subtracting the weight of Cu foil from that of the slurry-cast Cu foil and dividing it by the portion of the active material.
- Calculate the current at which the battery cell needs to be run by multiplying the current density (mA·g-1) with the weight of the active material.
- Insert the electrochemical battery cells in the battery cell tester. Apply the current (corresponding to 0.05 A·g-1 for the formation cycle and various current densities in the range of 0.1 A·g-1 to 10.0 A·g-1 for the cycle tests and rate capabilities) for each battery cell using the battery cell tester program.
- Apply different currents for each battery cell if it is tested at various current densities.
3. Preparation of the Graphene Liquid Cell
- Synthesize graphene by chemical vapor deposition (CVD).
- Cut Cu foil (purity: 99.9%, thickness: 0.0125 mm) with scissors into pieces with a dimension of 10 x 3 cm.
- Rinse the Cu foil from step 3.1.1 with isopropyl alcohol (IPA) to remove any dust or contaminants and treat it with 100 mL of 20 wt% phosphoric acid (H3PO4) for 20 min to remove native oxide on the surface of the Cu foil in a glass Petri dish. Then, place the Cu foil in DI water for another 10 min to fully rinse the remaining H3PO4.
- Move the Cu foil to the quartz tube (outer diameter: 40 mm, inner diameter: 36 mm) of the CVD equipment.
- Run the rotary pump and wait until the vacuum level is under 2 x 10-3 Torr. Then, elevate the temperature to 150 °C to fully remove the oxygen and moisture inside the quartz tube of the CVD.
- Elevate the temperature from RT to 1,000 °C in 40 min with 10 sccm of H2 gas flow. Maintain the temperature of the chamber for another 40 min to anneal the Cu foil.
- Turn on 60 sccm of CH4 gas for 25 min. Cool down the CVD chamber to RT. Turn the CH4 and H2 gases off at 300 °C.
- Take the Cu foil (Figure 2a) from the CVD chamber and keep it in a desiccator.
- Transfer the graphene.
- To remove the graphene on the backside of the Cu foil, conduct plasma etching by using a plasma cleaner with the following settings: a flow of Ar (of 100 sccm), time (of 60 s), power (of 30 W), and a base pressure (of 5.0 x 10-2 Torr).
- Cut the Cu foil with the graphene that was synthesized in step 3.1 to 3 x 3 mm with scissors. Place the Cu foil pieces between two slide glasses and press to make them flat.
NOTE: Four Cu foil pieces are placed together between two slide glasses. - Place holey carbon Au grids (300 mesh, R2/2) on each piece of Cu foil (Figure 2b). Drop 20 µL of IPA on the Au grid/Cu foil.
- Suction IPA with a micropipette tip that is connected to a rotary pump. After suctioning, dry the Au grid/Cu foil at 50 °C for 5 min.
- Conduct the etching of the Cu foil in 10 mL of 0.1 M ammonium persulfate for 6 h in a 6 cm glass Petri dish (Figure 2c).
NOTE: Glass Petri dishes must be cleaned with IPA and DI water prior to use in order to avoid the contamination of the Si particles. - Scoop the Au grids with a Pt loop and move it to a glass Petri dish filled with DI water at 50 °C, in order to fully remove any remaining contaminants from the etchant16.
- Scoop the Au grids from the DI water and dry them for 6 h at RT and with atmospheric pressure.
- Fabricate GLCs.
- Prepare the electrolyte and nanotube mixture. Disperse 0.06 g of nanotube powder in 10 mL of electrolyte, which is composed of 1.3 M lithium hexafluorophosphate (LiPF6) in ethylene carbonate (EC) and diethylene carbonate (DEC) (3:7 volume ratio) with 10 wt% of fluoroethylene carbonate (FEC).
NOTE: The composition of the electrolyte is the same as the one used in the electrochemical battery cell test. Various electrolytes can be employed in GLCs, such as 1 M LiPF6 dissolved in EC, DEC, and dimethyl carbonates (DMC) in a volumetric ratio of 1:1:1, 1 M of sodium hexafluorophosphate (NaPF6) dissolved in EC, 1 M of sodium perchlorate (NaClO4) dissolved in polyethylene carbonate (PC) with 5 wt% of FEC, 0.1 M of magnesium bis(trifluoromethanesulfonimide) (MgTFSI) in diglyme, and 1 M NaClO4 in PC. - Move the graphene-transferred Au grids and electrolyte mixture into a glove box that is filled with Ar.
- Place one graphene-transferred Au grid on the bottom. Drop 20 µL of electrolyte mixture on the bottom grid.
- Hold another graphene-transferred grid with a tweezer and place it on the top of the bottom grid.
NOTE: This procedure must be done quickly before the electrolyte has dried (Figure 2d). - Dry the sample inside the glove box for 30 min, during which the liquid is spontaneously encapsulated between the two graphene sheets as it dries.
NOTE: The amount of entrapped liquid depends on how well the graphene has been transferred and how well the upper grid is placed.
- Prepare the electrolyte and nanotube mixture. Disperse 0.06 g of nanotube powder in 10 mL of electrolyte, which is composed of 1.3 M lithium hexafluorophosphate (LiPF6) in ethylene carbonate (EC) and diethylene carbonate (DEC) (3:7 volume ratio) with 10 wt% of fluoroethylene carbonate (FEC).
4. Performing Real-time TEM
- Load GLCs on a conventional single-tilt TEM holder.
- Place the GLC sample (two attached graphene-transferred Au grids) on the single-tilt TEM holder.
- If the two grids are not stacked up perfectly, the GLC sample will not fit in the TEM holder. In that case, cut the edge of the Au grids with a razor blade.
- After the GLC sample is mounted on the TEM holder, put the TEM holder inside the TEM and carefully check the vacuum level.
- Record real-time TEM videos.
- Find the region where the SnO2 nanotube is encapsulated with liquid electrolyte.
NOTE: To find out whether liquid exists around the SnO2 nanotube, irradiate an electron beam for a few seconds. If some movement of liquid or the decomposition of an electrolyte is observed, it is highly likely that the area is encapsulated with liquid. - Do alignment for TEM and set the electron beam dosage to initiate the reaction by adjusting the brightness knob.
NOTE: The suitable alignment for TEM includes user alignment, such as Z-height alignment, gun tilt/shift, beam tilt/shift, aperture alignment, and stigmation alignment. These procedures are better done in another area (right next to the region found in step 4.2.1) in order not to give any damage to the SnO2 nanotube and liquid electrolyte. The electron beam dose rate for initiating the lithiation is usually ~103e–/Å2·s, but it may differ with every TEM instrument. - Run the microscopy program and charge-coupled device (CCD) camera according to the manufacturer's instructions.
- Press the record button on the high definition (HD) video window and record the reaction occurring in the GLC sample.
- Find the region where the SnO2 nanotube is encapsulated with liquid electrolyte.
Representative Results
SnO2 nanotubes were fabricated by electrospinning and subsequent calcination, during which the nanotubular and porous structures could be seen clearly, according to the SEM image (Figure 3a). Such a nanotubular structure comes from the decomposition of PVP, while the Sn precursor in the core is moved outward due to the Kirkendall effect17,18. Additionally, Ostwald ripening occurs in addition to the Kirkendall effect, resulting in the growth of SnO2 nanogains19. The TEM image (Figure 3b) shows that such porous sites are more visually clear, indicated by a number of white spots within the SnO2 nanotubes. The crystal structures of SnO2 are polycrystalline cassiterite structures (Figure 3c), in accordance with previously published literature17.
In terms of electrochemical characteristics of the SnO2 nanotubes, various aspects of the SnO2 nanotubes were examined in detail. To start with, the charge and discharge profile of the SnO2 nanotubes in the formation cycle is shown (Figure 4a), which exhibits stable voltage profiles with an initial coulombic efficiency of 67.8%. The voltage plateau, which exists at 0.9 V, can be attributed to the two-phase reaction (the conversion reaction of SnO2 to Sn), similar to descriptions in previous works9,20. The irreversible formation of Li2O during the conversion reaction of SnO2, along with the unstable formation of the solid electrolyte interphase (SEI) layer, resulted in a poorly reversible reaction with Li in the formation cycle. The SnO2 nanotubes exhibit stable cycling at 500 mA g-1, with coulombic efficiencies above 98% (Figure 4b). The rate capabilities of SnO2 nanotubes (Figure 4c) are also presented, where the SnO2 nanotubes retain considerable capacity (> 700 mAh g-1) even at a high current density of 1,000 mA g-1. Nevertheless, initial irreversible capacity loss needs to be examined more in detail using in situ TEM methods.
Overall characterizations of graphene are shown in Figure 5. Figure 5a shows the Raman spectrum of graphene synthesized on Cu foil. The ratio between Ig and I2D was 2.81, which matches well with the ratio of monolayer graphene on polycrystalline Cu substrate, indicating that monolayer graphene was synthesized. The SEM image of transferred graphene on an Au TEM grid is shown in Figure 5b, demonstrating that the coverage of graphene was good after its transfer to the Au TEM grid. The TEM image and the corresponding selected area electron diffraction (SAED) pattern of the transferred graphene are shown in Figure 5c,d. The hexagonal diffraction spots indicate the monolayer graphene well.
Time-series TEM images of GLCs are shown in Figure 6, which are captured from Movie S1. When GLCs are fabricated well, they have multiple liquid pockets whose sizes range from tens of nanometers to hundreds of nanometers, depending on the solution and nanoparticles7,14. In this experiment, using EC/DEC/FEC solution and SnO2 nanotubes, the size of the liquid pocket was 300 – 400 nm. The accelerating voltage was 300 kV and the electron beam dosage 743.9 e–/Å2·s, which is enough for lithiation to proceed but not for severe beam damage. Through constant electron beam irradiation, dissolved electrons and radicals trigger a secondary reaction with the salt and solvent. Here, the decomposition of electrolyte and the formation of an SEI layer were observed at the initial stage, in agreement with some of the previously reported on reuslts6,7,8,9,21.
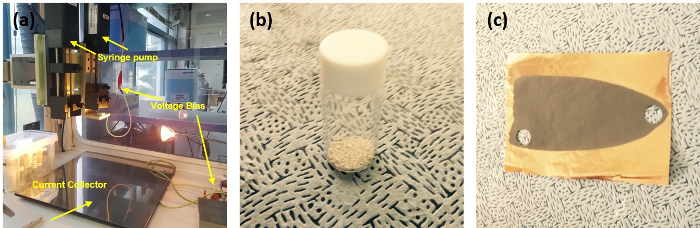
Figure 1: Digital camera images of the electrospinning setup and prepared SnO2 nanotubes and electrode. (a) Electrospinning, (b) SnO2 nanotubes, and (c) the slurry-cast electrode. Please click here to view a larger version of this figure.
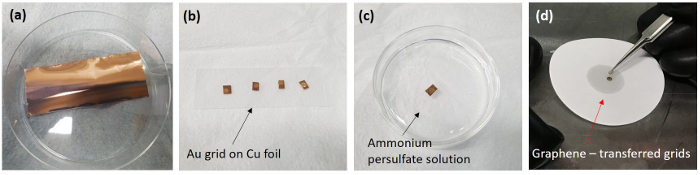
Figure 2: Digital camera images showing the graphene-transferred grid and fabrication of the graphene liquid cells. (a) The synthesized monolayer graphene on Cu foil, (b) an Au TEM grid on Cu foil, (c) the etching process of Cu foils in 0.1 M ammonium persulfate, and (d) stacked Au grids inside a glove box. Please click here to view a larger version of this figure.
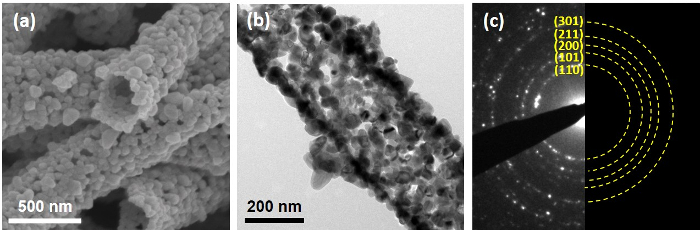
Figure 3: Characterization of SnO2 nanotubes before their encapsulation inside the graphene sheet. These panels show (a) an SEM image, (b) a TEM image, and (c) the SAED pattern of the SnO2 nanotubes. Please click here to view a larger version of this figure.
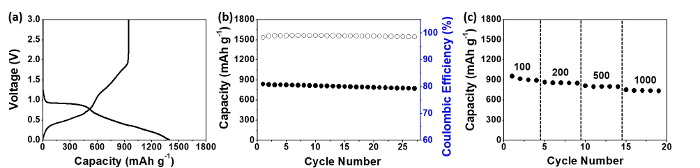
Figure 4: Electrochemical battery cell testing of the SnO2 nanotubes. These panels show (a) the charge and discharge profile, (b) the cycle retention characteristics, and (c) the rate capabilities of the SnO2 nanotubes. Please click here to view a larger version of this figure.
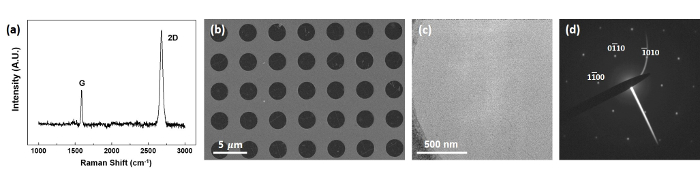
Figure 5: Characterization of synthesized graphene. These panels show (a) the Raman spectrum, (b) the SEM image, (c) the TEM image, and (d) the SAED pattern of the monolayer graphene. Please click here to view a larger version of this figure.

Figure 6: Real-time TEM images of the lithiation process of GLCs. Decomposed electrolyte and the formation of an SEI layer on the surface of an SnO2 nanotube are observed for 0 – 45 s. Please click here to view a larger version of this figure.
Movie S1. Lithiation of GLCs. The surface of an SnO2 nanotube is visualized inside liquid electrolyte. Please click here to view this video. (Right-click to download.)
Discussion
There are critical steps within the protocol. First, the transfer of the graphene onto the TEM grid needs the researchers' careful attention. It is important to handle the grids with tweezers and not damage any of grids, for instance by destroying the amorphous carbon membrane or bending the frame. These kinds of damages will result in a poor coverage of the graphene and affect the number of liquid pockets. In addition, placing the upper grid at the right position is critical. As described in the protocol, the top grid must be placed quickly before the liquid has dried. During this process, researchers may damage the upper grid or place it in the wrong position (i.e., not in the center of the bottom grid). Similar to any damages incurred during the transfer process, this will lower the yield of liquid cells. Thus, much practice with handling the TEM grids is needed to repeatedly fabricate GLCs.
It is important to make sure that the slurry-cast Cu foil has fully dried prior to the cell assembly. This is important because the presence of water can degrade the overall cell performance. Additionally, the slurry should be cast on the Cu foil uniformly, so that the loading amount of active material is similar. Moreover, it is important to find the right place for the TEM observation, where the liquid is completely sealed by the graphene sheets and enough liquid exists so that lithiation can continuously take place. Even though researchers followed the steps as they are demonstrated in the protocol, they will often observe incomplete reactions and depletion of liquid electrolyte around active materials. To find the right place for the TEM observation, the researchers should illuminate the electron beam for a few seconds and observe whether enough liquid exists for further reaction to occur.
The limitation of the GLC technique with observing lithiation is that the dynamics are possible only upon lithiation, not delithiation. Because lithiation inside GLCs is initiated by an electron beam and the reduction of surrounding electrolyte, the opposite oxidizing environment cannot be realized. This is a limitation compared to other in situ TEM techniques that can apply bias to the system, such as a scanning tunneling microscope (STM)-TEM holder or electrochemistry holders. Also, as two grids are attached and the upper grid is not removed in this experiment, aqueous solvents have less ability to stick two grids together and organic electrolyte is therefore preferred.
GLCs provide major advances in three different ways. 1) They provide high-resolution imaging in a liquid electrolyte that is hardly achievable in other in situ TEM platforms. 2) They do not require the purchase of an additional in situ TEM holder. 3) Also, various kinds of nanomaterials (such as nanosheet, nanoparticle, and nanofiber) can be visualized inside the liquid electrolyte.
GLCs can be used further to observe not only the dynamics of electrode materials upon lithiation but, also, sodiation (Na-ion batteries), magnesiation (Mg-ion batteries), potassiation (K-ion batteries), and zinc insertion (Zn-ion batteries). Furthermore, beyond the decomposition of various kinds of electrolytes, morphological changes of electrode materials can be visualized inside the GLC9,10. We expect that such information will provide valuable insights for engineers who are working on designing advanced secondary ion batteries.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF), grant no. 2014R1A4A1003712 (BRL Program), the Korea CCS R&D Center (KCRC) grant funded by the Korea government (Ministry of Science, ICT & Future Planning) (No. NRF-2014M1A8A1049303), an End-Run grant from KAIST funded by the Korea government in 2016 (Ministry of Science, ICT & Future Planning) (N11160058), the Wearable Platform Materials Technology Center (WMC) (NR-2016R1A5A1009926), an National Research Foundation of Korea (NRF) Grant funded by the Korean Government (NRF-2017H1A2A1042006-Global Ph.D. Fellowship Program), a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP; Ministry of Science, ICT & Future Planning) (NRF-2018R1C1B6002624), the Nano·Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, and an ICT and Future Planning (2009-0082580) and NRF grant funded by the Korea government (MSIP; Ministry of Science, ICT & Future Planning) (NRF-2018R1C1B6002624).
Materials
| Tin chloride dihyrate | Sigma Aldrich | CAS 10025-69-1 | In a glass bottle |
| Ethanol | Merck | CAS 64-17-5 | In a glass bottle |
| Dimethylformamide | Sigma Aldrich | CAS 68-12-2 | In a glass bottle |
| Polyvinylpyrrolidone | Sigma Aldrich | CAS 9003-39-8 | In a plastic bottle |
| Cell tester | KOREA THERMO-TECH | Maccor Series 4000 | |
| Cell tester 2 | WonaTech | WBCS4000 | |
| Sodium perchlorate | Sigma Aldrich | CAS 7601-89-0 | In a glass bottle |
| 25 gauge needle | Hwa-In Science Ltd. | ||
| 1.3 M of lithium hexafluorophosphate (LiPF6) dissolved in EC/DEC with 10 wt% of FEC | PANAX ETEC | In a stainless steel bottle | |
| Propylene carbonate | Sigma Aldrich | CAS 108-32-7 | In a glass bottle |
| Super P Carbon Black | Alfa-Aesar | CAS 1333-86-4 | In a glass bottle |
| Cell components (bottom cell, top cell, separator, gasket, spring, spacer) | Wellcos Corporation | ||
| Cell punch | Wellcos Corporation | ||
| Glove Box | Moisture Oxygen Technology (MOTEK) | ||
| Box Furnace | Naytech | Vulcan 3-550 | |
| Electrospinning device | NanoNC | ||
| Hydrofluoric acid | Junsei | 84045-0350 | 85% |
| Cu foil | Alfaaesar | 38381 | Copper Thinfoil, 0.0125mm thick, 99.9% |
| Holy carbon Au grid | SPI | Quantifoil R2/2 Micromachined Holey Carbon Grids, 300 Mesh Gold | Quantifoil R2/2 Micromachined Holey Carbon Grids, 300 Mesh Gold |
| Isoprophyl alchol | Sigmaaldrich | W292907 | 99.70% |
| Ammonium persulfate | Sigmaaldrich | 248614 | 98% |
| Transmission electron microscope (TEM) | JEOL | JEOL JEM 3010 | 300 kV |
| Chemical vapor depistion (CVD) | Scientech | ||
| Charge coupled device (CCD) | Gatan | Orius SC200 | |
| Plasma Cleaner | Femtoscience | VITA | |
| Electrospinning program | NanoNC | NanoNC eS- robot |
References
- Sun, Y. -. K., et al. Nanostructured high-energy cathode materials for advanced lithium batteries. Nature Materials. 11 (11), 942-947 (2012).
- Manthiram, A., Fu, Y., Chung, S. -. H., Zu, C., Su, Y. -. S. Rechargeable Lithium-Sulfur Batteries. Chemical Reviews. 114 (23), 11751-11787 (2014).
- Liu, X. H., Huang, J. Y. In situ TEM electrochemistry of anode materials in lithium ion batteries. Energy Environmental Science. 4 (10), 3844-3860 (2011).
- Xie, Z. -. H., Jiang, Z., Zhang, X. Review-Promises and Challenges of In Situ Transmission Electron Microscopy Electrochemical Techniques in the Studies of Lithium Ion Batteries. Journal of the Electrochemical Society. 164 (9), 2100-2123 (2017).
- Tripathi, A. M., Su, W. -. N., Hwang, B. J. In situ analytical techniques for battery interface analysis. Chemical Society Reviews. 47 (3), 736-851 (2018).
- Yuk, J. M., Seo, H. K., Choi, J. W., Lee, J. Y. Anisotropic lithiation onset in silicon nanoparticle anode revealed by in situ graphene liquid cell electron microscopy. ACS Nano. 8 (7), 7478-7485 (2014).
- Cheong, J. Y., et al. Growth dynamics of solid electrolyte interphase layer on SnO2 nanotubes realized by graphene liquid cell electron microscopy. Nano Energy. 25, 154-160 (2016).
- Lee, K., Shin, S., Degen, T., Lee, W., Yoon, Y. S. In situ analysis of SnO2/Fe2O3/RGO to unravel the structural collapse mechanism and enhanced electrical conductivity for lithium-ion batteries. Nano Energy. 32, 397-407 (2017).
- Chang, J. H., et al. Direct realization of complete conversion and agglomeration dynamics of SnO2nanoparticles in liquid electrolyte. ACS Omega. 2 (10), 6329-6336 (2017).
- Cheong, J. Y., et al. In Situ High-Resolution Transmission Electron Microscopy (TEM) Observation of SnNanoparticles on SnO2 Nanotubes Under Lithiation. Microscopy Microanalysis. 23 (6), 1107-1115 (2017).
- Cheong, J. Y., et al. Revisiting on the effect and role of TiO2 layer thickness on SnO2 for enhanced electrochemical performance for lithium-ion batteries. Electrochimica Acta. 258, 1140-1148 (2017).
- Hwa, Y., Seo, H. K., Yuk, J. M., Cairns, E. J. Freeze-Dried Sulfur-Graphene Oxide-Carbon Nanotube Nanocomposite for High Sulfur-Loading Lithium/Sulfur Cells. Nano Letters. 17 (11), 7086-7094 (2017).
- Yuk, J. M., et al. High-Resolution EM of Colloidal Nanocrystal Growth Using Graphene Liquid Cells. Science. 336 (6084), 61-64 (2012).
- Jeong, M., Yuk, J. M., Lee, J. Y. Observation of Surface Atoms during Platinum Nanocrystal Growth by Monomer Attachment. Chemistry of Materials. 27 (9), 3200-3202 (2015).
- Yuk, J. M., et al. Real-Time Observation of Water-Soluble Mineral Precipitation in Aqueous Solution by In situ High-Resolution Electron Microscopy. ACS Nano. 10 (1), 88-92 (2015).
- Wang, C., Qiao, Q., Shokuhfar, T., Klie, R. F. High-Resolution Electron Microscopy and Spectroscopy of Ferritin in Biocompatible Graphene Liquid Cells and Graphene Sandwiches. Advanced Materials. 26 (21), 3410-3414 (2014).
- Cheong, J. Y., Kim, C., Jang, J. S., Kim, I. -. D. Rational design of Sn-based multicomponent anodes for high performance lithium-ion batteries: SnO2@TiO2@reduced graphene oxide nanotubes. RSC Advances. 6 (4), 2920-2925 (2016).
- Mel, A. -. A., Nakamura, R., Bittencout, C. The Kirkendall effect and nanoscience: hollow nanospheres and nanotubes. Beilstein Journal of Nanotechnology. 6, 1348-1361 (2015).
- Cheong, J. Y., Kim, C., Jung, J. -. W., Yoon, K. R., Kim, I. -. D. Porous SnO2-CuO nanotubes for highly reversible lithium storage. Journal of Power Sources. 373, 11-19 (2018).
- Ao, X., et al. Porous Honeycomb-inspired design of ultrafine SnO2@C nanospheres embedded in carbon film as anode materials for high performance lithium- and sodium-ion battery. Journal of Power Sources. 359, 340-348 (2017).
- Abellan, P., et al. Probing the Degradation Mechanisms in Electrolyte Solutions for Li-Ion Batteries by in Situ Transmission Electron Microscopy. Nano Letters. 14, 1293-1299 (2014).

