Evaluating Virulence and Pathogenesis of Aeromonas Infection in a Caenorhabditis elegans Model
Summary
Here, we introduce three different experiments to study Aeromonas infection in C. elegans. Using these convenient methods, it is easy to evaluate the toxicity among and within Aeromonas species.
Abstract
The human pathogen Aeromonas has been clinically shown to cause gastroenteritis, wound infections, septicemia, and urinary tract infections. Most human diseases have been reported to be associated with four species of bacteria: Aeromonas dhakensis, Aeromonas hydrophila, Aeromonas veronii, and Aeromonas caviae. The model organism Caenorhabditis elegans is a bacterivore that provides an excellent infection model by which to study the bacterial pathogenesis of Aeromonas. Here, we introduce three different experiments to study Aeromonas infection using a C. elegans model, including survival, liquid toxicity, and muscle necrosis assays. The results of the three methods determining the virulence of Aeromonas were consistent. A. dhakensis was shown to be the most toxic among the 4 major Aeromonas species causing clinical infections. These methods are shown to be a convenient way to evaluate the toxicity among and within Aeromonas species and contribute to our understanding of the pathogenesis of Aeromonas infection.
Introduction
The human pathogen, Aeromonas, has been shown clinically to cause gastroenteritis, wound infections, septicemia, and urinary tract infections1,2. Most associated human diseases have been reported to be associated with four bacterial species: Aeromonas dhakensis, Aeromonas hydrophila, Aeromonas veronii, and Aeromonas caviae 2,3,4,5. Among Aeromonas infectious diseases, soft-tissue infections can cause severe morbidity and mortality in humans. Of note, muscle necrosis is the most severe form of soft tissue infection6. Observation of the survival and muscle necrosis of Caenorhabditis elegans after infection is a convenient method by which to speculate the toxicity of Aeromonas.
Scientists have already developed numerous model organisms to study bacterial infections. In previous studies, mice, zebrafishes, and nematodes were used as animal models to study the pathogenesis and virulence of Aeromonas6,7,8. Every animal model has its' advantages and applications. The model organism, Caenorhabditis elegans, is a bacterivorous nematode which intakes bacteria as foodnaturally. C. eleganshas developed a complicated innate immune system against bacterial infection over the course of its evolution. Under the stress of bacterial infection, C. elegans has been proven to be an excellent infection model to study the bacterial pathogenesis of Aeromonas6,7,9 and other pathogens like fungus10 and enterohaemorrhagic Escherichia coli O157:H711. However, there is still no publication that focuses on the methodology in using C. elegans as a model for studying the virulence of Aeromonas.
Here, we introduce three different experiments to study Aeromonas infection using C. elegans as an animal model: assays for survival, liquid toxicity, and muscle necrosis. These methods are a convenient way to evaluate the toxicity among and within Aeromonas species and improve the understanding of the pathogenesis of Aeromonas.
Protocol
1. Preparation of the Culture Medium
NOTE: See Table 1 for solution preparation.
- To prepare M9 medium12, dissolve 1.5 g of KH2PO4, 5.66 g of Na2HPO4, and 2.5 g of NaCl in 500 mL of deionized water. Autoclave at 121 °C for 20 min. Wait until cooled to room temperature and then add 0.5 mL of 1 M MgSO4 before first use.
- To prepare nematode growth medium (NGM)12, dissolve 3 g NaCl, 2.5 g bacterial peptone, and 20 g agar in 1 L of deionized water. Autoclave at 121 °C for 20 min. Wait until cooled to 55 °C in a water bath and then add 1 mL of 1 M CaCl2, 1 mL of 1 M MgSO4, 1 mL of 5% cholesterol in ethanol, and 25 mL of phosphate buffer. Swirl to mix well.
- Pour about 5 mL NGM into each 6 cm Petri dish. Avoid bubble production on the surface of plate. Leave plates at room temperature for 1 night and package to save at 4 °C. Bring back to room temperature before use.
- To prepare enriched NGM (ENGM), dissolve 3 g NaCl, 5 g bacterial peptone, 1 g yeast extract, and 30 g agar in 1 L of deionized water. Autoclave at 121 °C for 20 min. Wait until cooled to 55 °C in a water bath, and add 1 mL of 1 M CaCl2, 1 mL of 1 M MgSO4, 1 mL of 5% cholesterol in ethanol, and 25 mL of phosphate buffer. Swirl to mix well.
- Pour about 10 mL ENGM into each 9 cm Petri dish. Avoid bubble production on the surface of plate. Leave plates at room temperature for 1 night and package to save at 4 °C. Bring to room temperature before use.
- To prepare S medium12, mix 40 mL S Basal, 0.4 mL 1 M potassium citrate, 0.4 mL trace metals solution, 0.12 mL of 1 M CaCl2, 0.12 mL of 1 M MgSO4, 40 µL of 5% cholesterol in ethanol and 1 mL of 8 mM FudR before use.
2. Synchronization of C. elegans6,9,12
- Wash about 2,000 worms in the gravid-adult stage with 10 mL of sterile deionized water in a 15 mL tube. Wash out the bacteria 3x, keeping the worms at the bottom of the tube.
- Centrifuge the sample for 1 min at 500 x g to pull down the worms. Remove the supernatant, and keep the worms in 3.5 mL deionized water.
- Add 1 mL NaOCl (10–15%) and 0.5 mL 5 M KOH into the tube. Mix evenly by shaking for <6 min to lyse the worm bodies.
- After the eggs are released from the worms, add 10 mL deionized water to stop the lysis. Centrifuge for 1 min at 1,200 x g to pull down the eggs and remove the supernatant as much as possible. Wash with 15 mL M9 medium at least 3x.
NOTE: See step 1.1 for the composition of M9 medium. - Keep eggs in 1 mL M9 medium. Transport eggs to a 3.5 cm dish for incubation at 20 °C for one night (about 12–18 h).
NOTE: After the incubation, the eggs will hatch to worm larva which is called as first larva (L1) stage. - Pipette out 10 µL of L1 worms in M9 medium. Count the worm number and calculate the concentration of L1 worms in M9 medium.
- Seed at most 10,000 incubated L1 stage worms with a pipet on a 9 cm ENGM plate spread with Escherichia coli OP50.
- In this step, spread 0.5 mL overnight cultured E. coli OP50 with Luria-Bertani (LB) broth on the 9 cm ENGM plate. Incubate the plate at 37 °C for 16–18 h
NOTE: To avoid contamination, the spreading step should be performed in a laminar flow hood. - Cool to room temperature before seeding L1 worms. Seed at most 10,000 incubated L1 stage worms on the ENGM plate with E. coli OP50.
- In this step, spread 0.5 mL overnight cultured E. coli OP50 with Luria-Bertani (LB) broth on the 9 cm ENGM plate. Incubate the plate at 37 °C for 16–18 h
- Incubate the worms at 20 °C for 44 h or until they grow to the 4th larva (L4) stage.
NOTE: See step 1.3 for the composition of ENGM medium. A worm has a white dot in half-moon shape at the middle of body side at the L4 stage. - Use the synchronized L4 stage worms in the following assays. Use wild type N2 worms in the C. elegans survival assay with Aeromonas dhakensis and the C. elegans liquid toxicity assay with Aeromonas dhakensis. Use RW1596 worms (myo-3(st386);stEx30[myo-3p::GFP::myo-3 + rol-6(su1006)]) in the C. elegans muscle necrosis assay with Aeromonas dhakensis.
3. C. elegans Survival Assay with Aeromonas6,9
- On the day of seeding L1 worms on the ENGM spreading with E. coli OP50, pick a single colony of each of the four Aeromonas strains or E. coli OP50 and culture with 2 mL LB broth respectively at 37 °C for 16 h.
NOTE: To avoid contamination, this step should be performed in a laminar flow hood. Here, the following bacterial strains were used: E. coli OP50, the normal food source of C. elegans. A. dhakensis AAK1, the first fully-sequenced pathogenic clinical A. dhakensis isolate. A. hydrophila A2-066, A. veronii A2-007, and A. caviae A2-9307121 are representatively clinical isolates from National Cheng Kung University Hospital. - Measure the OD600 absorbance of bacterial broth and adjust the bacterial broth to OD600 = 2.0 with LB broth.
- Spot and spread 30 µL of bacterial broth each 6 cm NGM plate. Culture the plates at room temperature overnight.
NOTE: See step 1.2 for the composition of NGM medium. - On the day the L1 worms grow to the L4 stage, randomly pick and transfer 50 worms to an NGM plate with each of the four Aeromonas strains or E. coli OP50.
- Incubate the NGM plates at 20 °C until the assay is finished.
- Transfer all the living worms to a newly prepared NGM plate with bacteria and count the numbers of live, dead, and sensor worms every day until the last worm is dead.
NOTE: Transfer worms to a fresh-prepared NGM plate every day for the maintenance of infection, even if using sterile worms (e.g., glp-4, or with FudR). - Plot the survival curves based on the daily data calculation.
4. C. elegans Liquid Toxicity Assay with Aeromonas7
NOTE: To avoid contamination, steps should be performed in a laminar flow hood.
- The day after seeding the L1 worms on the ENGM plate spread with E. coli OP50, pick a single colony of each of the four Aeromonas strains and E. coli OP50, and culture with 5 mL LB broth each at 37 °C for 16 h.
NOTE: In the protocol, the following bacteria strains were used: E. coli OP50, the normal food source of C. elegans. A. dhakensis AAK1, the first fully-sequenced pathogenic clinical A. dhakensis isolate. A. hydrophila A2-066, A. veronii A2-007, and A. caviae A2-9307121 are representatively clinical isolates from National Cheng Kung University Hospital. - Measure the OD600 absorbance of the bacteria broth.
- Centrifuge the bacterial broth at 3,500 x g for 15 min to remove the LB broth. Resuspend the bacteria and adjust the bacterial broth to OD600 = 3.0 with S medium.Add 195 µL of bacterial broth in S medium to at least 8 wells of a 96-well plate.
NOTE: See step 1.6 for the composition of S medium. - Wash off the L4 worms on the ENGM plate with E. coli OP5 using M9 medium. Adjust the worm solution to a concentration of 5 worms per µL and add 5 µL of the worm solution to each well of the 96-well plate. Ensure that there are approximately 25 worms per well.
- Incubate the 96-well plate on a shaker at 200 rpm at 25 °C.
- Count the number of live worms and dead worms after 24, 48, and 72 h. Calculate the survival rates of each well with the following formula: (Live worms/Total worms) × 100%.
- Draw a scatter plot graph with the daily data calculation.
5. C. elegans Muscle Necrosis Assay with Aeromonas6
NOTE: To avoid contamination, these steps should be performed in a laminar flow hood.
- On the day the L1 worms are seeded on the ENGM spread with E. coli OP50, pick a single colony of each of the four Aeromonas strains and E. coli OP50 and culture with 2 mL LB broth each at 37 °C for 16 h.
NOTE: Here, the following bacteria strains were used: E. coli OP50, the normal food source of C. elegans. A. dhakensis AAK1, the first fully-sequenced pathogenic clinical A. dhakensis isolate. A. hydrophila A2-066, A. veronii A2-007, and A. caviae A2-9307121 are representatively clinical isolates from National Cheng Kung University Hospital. - Measure the OD600 absorbance of the bacteria broth and adjust the bacteria broth to OD600 = 2.0 with LB broth. Spot and spread 30 µL of bacterial broth on 6 cm NGM plates. Culture the plates at room temperature overnight.
- On the day the L1 worms grow to the L4 stage, transfer 50 worms to each NGM plate with each of the four Aeromonas strains or E. coli OP50. Incubate the NGM plates at 20 °C.Transfer worms every 24 h to newly prepared NGM plates with bacteria.
- Take muscle images using a fluorescence microscope.
- Randomly pick 10 worms onto a 2% agarose gel in M9 medium on a slide. Paralyze the worms using 2 µL of 1% sodium azide in M9 medium on agarose for less than 5 min before taking images.
- Place a cover slip and capture the muscle images using a green fluorescent protein (GFP) filter on a fluorescent microscope with charge-coupled device camera. Capture the muscle images at 24, 48, and 72 h post the L4 stage.
- Conduct image analysis and figure preparation with an image processing software.
NOTE: The definitions of the scores and the muscle damage levels are shown in Figure 3, for which the criteria are listed as follows: 3 points for missing muscle fibers; 2 points for ruptured or broken muscle fibers; 1 point for bent muscle fibers; 0 points for healthy muscle fibers.
6. Statistical Analyses
- Perform all experiments a minimum of three times independently.
- Use the Kaplan-Meier method to assess the C. elegans survival assays, and use the log-rank test in analyzing survival differences. Set statistical significance at 0.05.
- Use Student's t-test to analyze the statistical results of the C. elegans liquid toxicity assays for the two different groups. Use one-way ANOVA test in analyzing differences among three or more values for one independent variable. Set statistical significance at 0.05.
Representative Results
By following the protocols described above, it is easy to differentiate between the toxicities from the four Aeromonas strains. The survival assay of C. elegans is shown in Figure 1. The survival rates of C. elegans infected with Aeromonas species, shown in order from high to low were: A. caviae, A. veronii, A. hydrophila, and A. dhakensis. Although there is diversity in terms of toxicity among and within Aeromonas species, the survival rates of C. elegans, on average, showed highest mortality among the worms infected with A. dhakensis. The virulence of A. hydrophila was inferior to that of A. dhakensis (p < 0.01). In contrast to A. dhakensis and A. hydrophila,the attenuated virulences of both A. veronii and A. caviae were observed in the C. elegans survival assay. The diversity of the survival rates of C. elegans infected with different Aeromonas species is consistent with clinical observations of Aeromonas infection7.
The results of the liquid toxicity assay of C. elegans are shown in Figure 2. With the infection time, the numbers of worms that survived decreased significantly when C. elegans was infected with either A. dhakensis or A. hydrophila. The survival rate of the worms infected with A. dhakensis was inferior to those infected with A. hydrophila. In contrast, the groups infected with A. veronii or A. caviae had higher survival rates at each time point as compared to A. dhakensis and A. hydrophila. The trends of survival rates of C. elegans in the liquid toxicity assay representing the virulence of different Aeromonas species are similar to those observed in the survival assay.
In the C. elegans muscle necrosis assay, we first classified the levels of muscle damage and assigned scores according to the corresponding levels. The definitions of the score and the muscle damage levels are provided in Figure 3. The scoring criteria for each muscle damage level is as follows: 0 points for healthy muscle fibers; 1 point for bent muscle fibers; 2 points for ruptured or broken muscle fibers; 3 points for loss of muscle fibers. The results of the C. elegans muscle necrosis assay in Aeromonas infected-C. elegans is shown in Figure 4. The degrees of muscle damage varied among the Aeromonas species. The levels of muscle damage ranged in order from mild to severe as follows: A. caviae, A. veronii, A. hydrophila, and A. dhakensis at each time point. In contrast with A. hydrophila and A. dhakensis, muscle necrosis was not obvious in worms infected with A. caviae and A. veronii. Most of the worms infected with A. caviae and A. veronii were recorded as 0 points. C. elegans infected with A. hydrophila or A. dhakensis exhibited severe muscle damage. Of note, most of the worms infected with A. dhakensis demonstrated muscle damage graded ≥2 points. The degree of muscle necrosis of C. elegans infected with A. dhakensis was more severe in terms of a higher percentage of scores ≥ 2 points compared with A. hydrophila. The severity of muscle necrosis in the 4 Aeromonas species correlated with the findings from the survival and liquid toxicity assays.
The methods described above offer a convenient way to differentiate virulence diversity among and within Aeromonas species. In addition, this is a reliable model by which to study the interaction between a pathogen and host.
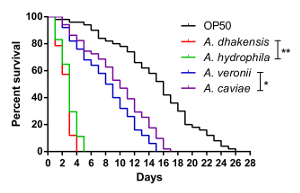
Figure 1: The survival curves of C. elegans. The survival assay shows the diverse toxicities of different Aeromonas species to C. elegans (**: P < 0.01; *: P < 0.05). Please click here to view a larger version of this figure.
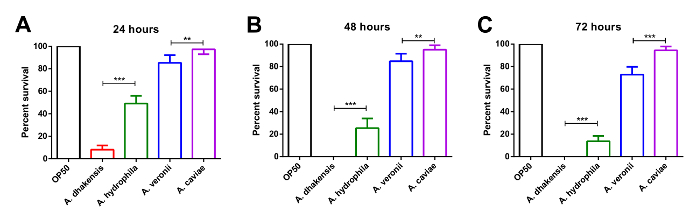
Figure 2: The survival rate of C. elegans from the liquid toxicity assay. The liquid toxicity assay shows the different toxicities of various Aeromonas species to C. elegans. At (A) 24 h, (B) 48 h, and (C) 72 h post Aeromonas infection, C. elegans infected with A. dhakensis has the lowest survival rate. The result suggests that A. dhakensis is the most toxic Aeromonas species to C. elegans (***: P < 0.001; **: P < 0.01, Error bars: standard deviation, SD). Please click here to view a larger version of this figure.
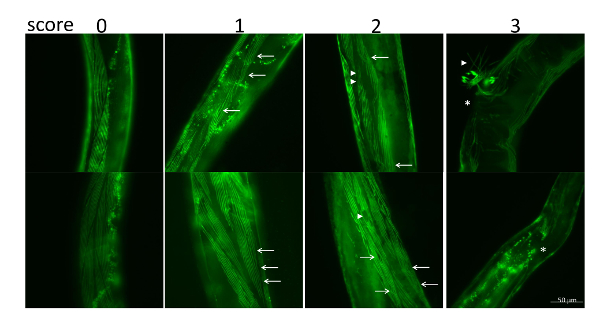
Figure 3: The corresponding scores for muscle necrosis in C. elegans induced by Aeromonas infection. The criteria for muscle necrosis and corresponding scores are as follows: 3 points for missing muscle fibers; 2 points for ruptured or broken muscle fibers; 1 point for bent muscle fibers; 0 points for healthy muscle fibers. Bent muscle fibers (arrows), ruptured muscle fibers (arrowheads), and missing muscle fibers (star) are indicated. Please click here to view a larger version of this figure.
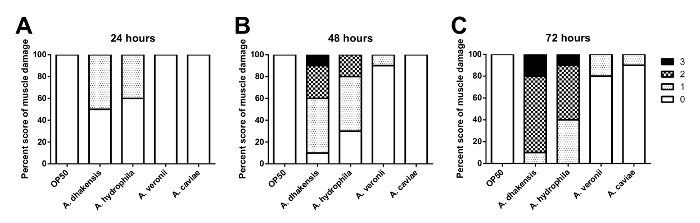
Figure 4: The C. elegans muscle necrosis scores. The muscle necrosis scores show the various toxicities of different Aeromonas species to C. elegans. At (A) 24 h, (B) 48 h, and (C) 72 h post Aeromonas infection, the C. elegans muscle damage is the severest from infection with A. dhakensis. Please click here to view a larger version of this figure.
| Solution | Preparation |
| Phosphate Buffer | Dissolve 119.35 g of KH2PO4 and 21.43 g of K2HPO4, in 1 L deionized water. Autoclave at 121 °C for 20 min Adjust the pH value to pH = 6.0 with KOH. Wait until cooled to room temperature |
| S Basal | Dissolve 5.85 g NaCl, 6 g KH2PO4, and 1 g K2HPO4 in 1 L of deionized water. Autoclave at 121 °C for 20 min. Wait until cooled to room temperature. |
| 1 M Potassium citrate | Dissolve 4 g citric acid•H2O and 58.7 g tri-potassium citrate•H2O in 0.2 mL of deionized water. Adjust the pH value to pH=6.0. Autoclave at 121 °C for 20 min. Wait until cooled to room temperature. |
| Trace metals solution | Dissolve 1.86 g disodium EDTA, 0.69 g FeSO4•7 H2O, 0.2 g MnCl2•4 H2O, 0.29 g ZnSO4•7 H2O, 0.025 g CuSO4•5 H2O in 1 L deionized water. Autoclave at 121 °C for 20 min. Wait until cooled to room temperature Keep the solution in the dark. |
| LB broth | Dissolve 10 g NaCl, 10 g tryptone, and 5 g yeast extract in 1 L of deionized water. Autoclave at 121 °C for 20 min. Wait until cooled to room temperature. |
Table 1: Solution Preparation.
Discussion
C. elegans is a bacterivorous nematode that naturally intakes bacteria as food and has developed a complicated innate immunity to bacteria during its evolutionary process. Two of the major organs maintaining and supporting the immunity are the epidermis and intestine9,13. The epidermis and bands of muscle of C. elegans resemble the soft-tissue structures in mammals and humans6. Because of these characteristics, C. elegans is applicable as a model organism for studying the pathogenesis of Aeromonas infection.
Here, we present three methods for examining Aeromonas infection in C. elegans that are representative of the diverse toxicity among 4 Aeromonas species and are correlated with clinical observation7. These convenient methods distinguish the different degrees of toxicity among the Aeromonas species. Using these methods, researchers can study the virulence of different Aeromonas species and the defending mechanism of hosts upon Aeromonas infection.
In these three methods, C. elegans acts not only as a predator but also as a prey of Aeromonas. If the worms are not in healthy condition before test, the results of host-pathogen interaction will be different. Thus, it is important to be very careful when manipulating C. elegans. To avoid excessive damage to the eggs of C. elegans in the synchronizing step, be sure to observe the release of eggs continuously. Once the eggs are damaged, the hatching number of C. elegans eggs will largely decrease. Even if the damaged eggs hatch successfully, the worms are likely to have developmental problems. Therefore, it is critical to complete step 2.3 within 6 min after the worms are exposed to NaOCl and KOH.
The three methods described above show cohesive results on the Aeromonas toxicities to C. elegans, including two different experiments measuring the survival rates of C. elegans under Aeromonas infection and one observing the morphological change of muscle. The virulence test using different designed methods may provide diverse results. For example, scientists have previously observed different outcomes in C. elegans plate and liquid assays14. In our research, we also found there was discrepancy existing between these two assays9. It is believed that the external environment will influence virulence of the pathogen, and the researchers need to find the optimal conditions to explore the virulence mechanism. From another perspective, it is worth studying and understanding the underlying mechanisms in specific condition of infection.
In this work, we established an infection model in C. elegans to study the virulence of Aeromonas and the corresponding host response after infection. These three methods could be applied to study the other Aeromonas species beyond the 4 species addressed in the present study. In addition, these approaches will provide a platform to study the underlying mechanism responsible for muscle necrosis induced by bacterial infection and to develop potential therapies to improve the clinical outcomes of severe soft tissue infections.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We are grateful for the assistance from the C. elegans core facility in Taiwan and to the Diagnostic Microbiology and Antimicrobial Resistance Laboratory of National Cheng Kung University Hospital for providing the Aeromonas isolates. We also acknowledge the Caenorhabditis Genetics Center (CGC), and the WormBase. We also thank Savana Moore for editing the manuscript.
This study was partially supported by grants from the Ministry of Science and Technology of Taiwan (MOST 105-2628-B-006-017-MY3) and the National Cheng Kung University Hospital (NCKUH-10705001) to P.L. Chen.
Materials
| Shaker incubator | YIH DER | LM-570R | Bacteria incubation |
| K2HPO4 | J.T.Baker | MP021519455 | Culture medium preparation |
| KH2PO4 | J.T.Baker | 3246-05 | Culture medium preparation |
| Na2HPO4 | J.T.Baker | MP021914405 | Culture medium preparation |
| NaCl | SIGMA | 31434 | Culture medium preparation |
| MgSO4 | SIGMA | M7506 | Culture medium preparation |
| agar | Difco | 214530 | Culture medium preparation |
| CaCl2 | SIGMA | C1016 | Culture medium preparation |
| cholesterol | SIGMA | C8503 | Culture medium preparation |
| ethanol | SIGMA | 32205 | Culture medium preparation |
| KOH | SIGMA | P5958 | Culture medium preparation |
| 6 cm petri plate | ALPHA PLUS | 46 | agar plate preparation |
| 96-well plate | FALCON | 353072 | liquid assay |
| bacterial peptone | Affymetrix/USB | AAJ20048P2 | Culture medium preparation |
| yeast extract | SIGMA | 92144 | Culture medium preparation |
| citric acid•H2O | SIGMA | C1909 | Culture medium preparation |
| tri-potassium citrate•H2O | SIGMA | 104956 | Culture medium preparation |
| FudR | SIGMA | 1271008 | Culture medium preparation |
| disodium EDTA | SIGMA | E1644 | Culture medium preparation |
| FeSO4•7 H2O | SIGMA | 215422 | Culture medium preparation |
| MnCl2•4 H2O | SIGMA | 221279 | Culture medium preparation |
| ZnSO4•7 H2O | SIGMA | 204986 | Culture medium preparation |
| CuSO4•5 H2O | SIGMA | C8027 | Culture medium preparation |
| tryptone | SIGMA | 16922 | Culture medium preparation |
| Microscope system | Nikon | Eclipase Ti inverted | microscope imaging |
| Scientific CCD Camera | QImaging | Retiga-2000R Fast 1394 | microscope imaging |
References
- Parker, J. L., Shaw, J. G. Aeromonas spp. clinical microbiology and disease. Journal of Infection. 62 (2), 109-118 (2011).
- Chao, C. M., Lai, C. C., Tang, H. J., Ko, W. C., Hsueh, P. R. Skin and soft-tissue infections caused by Aeromonas species. European Journal of Clinical Microbiology & Infectious Diseases. 32 (4), 543-547 (2013).
- Chao, C. M., Lai, C. C., Tang, H. J., Ko, W. C., Hsueh, P. R. Biliary tract infections caused by Aeromonas species. European Journal of Clinical Microbiology & Infectious Diseases. 32 (2), 245-251 (2013).
- Chuang, H. C., et al. Different clinical characteristics among Aeromonas hydrophila, Aeromonas veronii biovar sobria and Aeromonas caviae monomicrobial bacteremia. Journal of Korean Medical Science. 26 (11), 1415-1420 (2011).
- Chao, C. M., Lai, C. C., Gau, S. J., Hsueh, P. R. Skin and soft tissue infection caused by Aeromonas species in cancer patients. Journal of Microbiology, Immunology and Infection. 46 (2), 144-146 (2013).
- Chen, P. L., et al. A Disease Model of Muscle necrosis caused by Aeromonas dhakensis infection in Caenorhabditis elegans. Frontiers in Microbiology. 7, 2058 (2016).
- Chen, P. L., et al. Virulence diversity among bacteremic Aeromonas isolates: ex vivo, animal, and clinical evidences. PLoS One. 9 (11), 111213 (2014).
- Saraceni, P. R., Romero, A., Figueras, A., Novoa, B. Establishment of infection models in zebrafish larvae (Danio rerio) to study the pathogenesis of Aeromonas hydrophila. Frontiers in Microbiology. 7, 1219 (2016).
- Chen, Y. W., Ko, W. C., Chen, C. S., Chen, P. L. RIOK-1 Is a Suppressor of the p38 MAPK Innate Immune Pathway in Caenorhabditis elegans. Frontiers in Immunology. 9, 774 (2018).
- Powell, J. R., Ausubel, F. M. Models of Caenorhabditis elegans infection by bacterial and fungal pathogens. Methods in Molecular Biology. 415, 403-427 (2008).
- Chou, T. C., et al. Enterohaemorrhagic Escherichia coli O157:H7 Shiga-like toxin 1 is required for full pathogenicity and activation of the p38 mitogen-activated protein kinase pathway in Caenorhabditis elegans. Cellular Microbiology. 15 (1), 82-97 (2013).
- Stiernagle, T. Maintenance of C. elegans. WormBook. , 1-11 (2006).
- Engelmann, I., Pujol, N. Innate immunity in C. elegans. Advances in Experimental Medicine and Biology. 708, 105-121 (2010).
- Feinbaum, R. L., et al. Genome-wide identification of Pseudomonas aeruginosa virulence-related genes using a Caenorhabditis elegans infection model. PLoS Pathogens. 8 (7), 1002813 (2012).

