Large Area Substrate-Based Nanofabrication of Controllable and Customizable Gold Nanoparticles Via Capped Dewetting
Summary
This protocol details a novel nano-manufacturing technique that can be used to make controllable and customizable nanoparticle films over large areas based on the self-assembly of dewetting of capped metal films.
Abstract
Recent scientific advances in the utilization of metallic nanoparticle for enhanced energy conversion efficiency, improved optical device performance, and high-density data storage have demonstrated the potential benefit of their use in industrial applications. These applications require precise control over nanoparticle size, spacing, and sometimes shape. These requirements have resulted in the use of time and cost intensive processing steps to produce nanoparticles, thus making the transition to industrial application unrealistic. This protocol will resolve this issue by providing a scalable and affordable method for the large-area production of nanoparticle films with improved nanoparticle control compared to the current techniques. In this article, the process will be demonstrated with gold, but other metals can also be used.
Introduction
Large-area nanoparticle film fabrication is critically important for the adoption of recent technological advances in solar energy conversion and high-density data storage with the use of plasmonic nanoparticles1,2,3,4,5. Interestingly, it is the magnetic properties of some of these plasmonic nanoparticles, which provide these nanoparticles with the ability to manipulate and control light at the nanoscale. This controllability of light provides the possibility to enhance light entrapment of the incident light at the nanoscale and increase the absorptivity of the surface. Based on these same properties and having the ability to have nanoparticles in either a magnetized and a non-magnetized state, scientists are also defining a new platform for high-density digital data storage. In each of these applications, it is critical that a large area and affordable nanofabrication technique is developed that allows for the control of nanoparticle size, spacing, and shape.
The available techniques to produce nanoparticles are mostly based on nanoscale lithography, which have significant scalability and cost issues. There have been multiple different studies that have attempted to address the scalability problem of these techniques, but to date, no process exists that provides the level of control needed for nanoparticle fabrication and is cost and time effective enough for adoption in industrial applications6,7,8,9,10,11. Some recent research efforts improved the controllability of pulsed laser induced dewetting (PLiD) and templated solid-state dewetting12,13,14, but they still have significant required lithography steps and thus the scalability problem.
In this manuscript, we present the protocol of a nanofabrication method that will address this scalability and cost issue that has plagued the adoption and use of nanoparticle films in widespread industrial applications. This processing method allows the control over the produced nanoparticle size and spacing by manipulating the surface energies which dictate the self-assembly of the nanoparticles formed. Here, we demonstrate the use of this technique using a thin gold film to produce gold nanoparticles, but we have recently published a slightly different version of this method using a nickel film and thus this technique can be used with any desired metal. The goal of this method is to produce nanoparticle films while minimizing the cost and complexity of the process and thus we have modified our previous approach, which used atomic layer deposition and nanosecond laser irradiation on a Ni-alumina system and replaced them with physical vapor deposition and a hot plate. The result of our work on a Ni-alumina system also showed an acceptable level of control on the morphology of the surface after the dewetting15.
Protocol
NOTE: The large-area fabrication of controllable and customizable gold nanoparticle films is achieved by following the detailed protocol. The protocol follows three major areas that are (1) substrate preparation, (2) dewetting and etching, and (3) characterization.
1. Substrate Preparation
- Clean the substrate (100 nm SiO2 on Si) using an acetone rinse followed by an isopropyl alcohol rinse and then dry using a stream of N2 gas.
- Load the substrate into the thermal evaporator system and evacuate to reach the desired pressure for the deposition of the metal film. Ensure that the chamber is evacuated to a pressure on the order of 10-6 Torr for the removal of air and water vapor in the chamber.
- Using the thermal evaporator, deposit the gold film at the desired thickness (5 nm in this case). The gold source material was obtained in the form of 0.5 mm diameter wire of gold (99.99% pure). Note that the thickness control for all the deposition stages is performed by the calibration of the machine, considering all important parameters and post measurement of the thickness. At both deposition stages, the argon pressure is a couple of millitorrs (1-5 mTorr), and the range is given as different pressures are chosen to calibrate for the deposition rate.
- Vent and remove the substrate with deposited metal film from the thermal evaporator system. The protocol can be paused here.
- Load the substrate with deposited metal film into the direct current (DC) magnetron sputter deposition system and evacuate to reach the desired pressure for deposition of the capping film (Table of Materials).
- To locate the sample in the machine, put the sample in the load lock and the device transfers the sample to the main deposition chamber to ensure a sufficient level of vacuum. Note that the deposition of the alumina capping layer tales place in the next step and this step is explaining the process of placing the sample in the apparatus and how the sample is transferred to the main deposition chamber.
- Deposit the capping layer of the desired material and thickness. Note that the deposition of the alumina follows a similar procedure and condition of the gold layer deposition, variable thickness alumina in this case. The alumina source material was obtained in the form of a 50.8 mm diameter, 6.35 mm thick sputter target of aluminum oxide (99.5% pure).
- Vent the DC magnetron sputter deposition chamber and remove the prepared sample. (Table of Materials). The protocol can be paused here.
2. Dewetting and Etching
- Place the prepared sample onto a pre-heated hot plate. For the 5 nm gold film capped with alumina, heat the sample at 300 °C and allow the sample to dewet for 1 h. The protocol can be paused here.
- Etch the alumina while leaving the gold and underlying SiO2/Si substrate with a 3:1:1=H2O:NH4OH:H2O2 (in wt%) solution at 80 °C for 1 h. Note that the process is performed in a hood and all precautions for dealing with corrosive and environmental hazardous material should be taken. The protocol can be paused here.
3. Characterization
- Prepare the sample to be vacuum compatible by rinsing with acetone and isopropyl alcohol followed by drying with N2.
- Image the nanoparticle films using scanning electron microscopy (SEM) under high vacuum and at high magnification (50,000X magnification in this case to resolve the minimum sized nanoparticles). The protocol can be paused here.
- Perform image analysis to obtain information of nanoparticle size and spacing distributions. The image analysis is done using a MATLAB-based code that thresholds the grayscale image, performs noise reduction and particle filling routines15.
Representative Results
The protocol described here has been used for multiple metals and has shown the ability to produce nanoparticles on a substrate over large-area, with controllable size and spacing. Figure 1 shows the protocol with representative results showing the ability to control the fabricated nanoparticle size and spacing. When following this protocol, the result, which is the fabricated nanoparticle film with size and spacing distributions, will be dependent upon the choice of metal, the choice of substrate, the choice of capping layer material, the metal thickness, and the capping layer thickness. By adjusting any one of these parameters, a shift and change in these distributions would be expected. As an example, the 5 nm gold film on SiO2 with a Al2O3 capping layer of thicknesses of 0 nm, 5 nm, 10 nm, and 20 nm result in average nanoparticle radii of 14.2 nm, 18.4 nm, 17.3 nm, and 15.6 nm, respectively, an average nanoparticle spacing of 36.9 nm, 56.9 nm, 51.3 nm, and 47.2 nm, respectively.
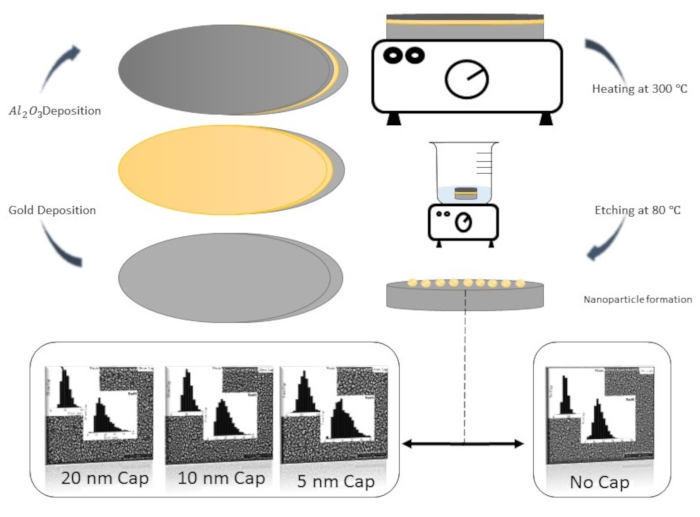
Figure 1: Graphical image of the protocol and representative results. The histograms presented are the pith (Top left) and Radii (Bottom left) distribution of the particle. Please click here to view a larger version of this figure.
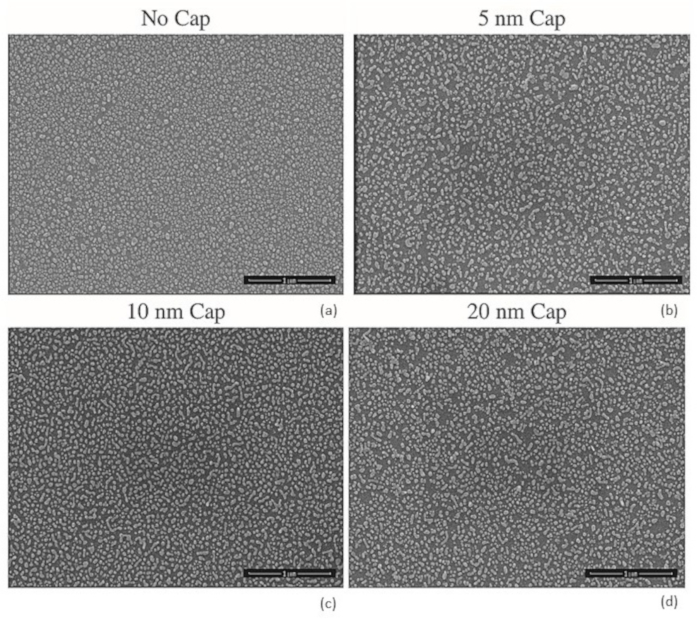
Figure 2: The SEM image of No-capping layer (a) and samples with 5 (b), 10 (c) and 20 nm (d) capping layer. The change in particle sizes and distributions are apparent comparing the images. Please click here to view a larger version of this figure.
Discussion
The protocol is a feasible and easy process for a nano-manufacturing process for producing nanoparticles on a substrate over large areas with controllable characteristics. The dewetting phenomenon, which leads to the production of particles, is based on the tendency of the dewetted layer to achieve minimum surface energy. The control over the size and shape of the particles is targeted with the deposition of a second surface on the main layer to tune the surface energies, and the final equilibrium between the adhesion and the energy required to bend the capping layer on the particles determines different dewetting regimes, which lead to different surface morphologies. This protocol has been designed and demonstrated based on equipment and processes that are typically accessible to anyone with basic microfabrication equipment and process capabilities. In the demonstrated approach, additional control over the final nanoparticle distribution can be achieved by changing the metal film thickness, the cap layer thickness, the substrate material, and the cap layer material. Between these process variables, a wide range of nanoparticle size and spacing can be achieved.
Adding additional steps or substituting techniques used in the current protocol can provide additional modification of the process resulting in more control over the nanoparticle distributions, including wider range of nanoparticle size and spacing, narrowing of the nanoparticle distributions, or the ability to produce multimodal nanoparticle films. This protocol was designed and demonstrated with an emphasis on accessibility and low cost. If more range is desired, the use of a rapid thermal annealing system or laser irradiation will change the heating rate and provide more nanoparticle control. If a multimodal nanoparticle distribution is desired, intermediate steps of lithography (electron beam lithography or photolithography) can be added before metal deposition or before cap layer deposition. The lithography step(s) will result in a variable thickness metal or cap layer across the surface and thus a different nanoparticle distribution.
Another modification that can be made easily is in the desired metal, depending on the specific application of the nanoparticle film. Here, the demonstration used gold because of the plasmonic properties, but similarly, a metallic nanoparticle or other plasmonic nanoparticle, or even a core-shell nanoparticle could be desired. This is achieved by changing the metal film material. This change will affect the resulting nanoparticle distribution because of the differences in surface energies, but the same trends would be expected. Note that the thickness of the capping layer provides control over the resulting nanoparticle size and spacing. For new material systems, an understanding of the extent of control will be needed.
This protocol was designed to eliminate the issue of large-area substrate-based nanoparticle fabrication for applications ranging from solar energy conversion to high-density data storage. These applications require a large-area of nanoparticles with well-defined and controlled nanoparticles. The techniques that are used in research labs to study the impact that nanoparticles have in these applications involved expensive equipment and time intensive processes, making them unfeasible for industrial applications. This protocol has demonstrated the level of control needed based on affordable and fast processing steps.
This protocol has the potential to be a revolutionary technique for the production of any nanoparticle films that require substrate-based processing. This demonstration was only done with a single material system, but more research will be done in the short term to explore the full capabilities of control and customization that is provided by this protocol.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We acknowledge the support from the Microscopy Core Facility at Utah State University for the SEM result. We also acknowledge the National Science Foundation (Award #162344) for the DC Magnetron Sputtering System, the National Science Foundation (Award #133792) for the (Field Electron and Ion) FEI Quanta 650, and the Department of Energy, Nuclear Energy University Program for the FEI Nova Nanolab 600.
Materials
| 100 nm SiO2/Si Substrate | University Wafer | Thermal Oxide Wafer | |
| Alumina Sputter Target (99.5%) | Kurt J. Lesker | Alumina Target | |
| Gold Wire (99.99%) | Kurt J. Lesker | Gold Wire | |
| H2O2 | Sigma-Aldrich | ||
| Hot Plate | Thermo Scientific | Cimarec | |
| NH4OH | Sigma-Aldrich | ||
| Scanning Electron Microscope | FEI | Quanta 650 | |
| Scanning Electron Microscope | FEI | Nova Nanolab 600 | |
| Sputter Deposition System | AJA International | Orion-5 | |
| Thermal Evaporator | Edwards | 360 |
References
- Pillai, S., Catchpole, K. R., Trupke, T., Green, M. A. Surface plasmon enhanced silicon solar cells. Journal of Applied Physics. 101 (9), 093105 (2007).
- Ding, B., Lee, B. J., Yang, M., Jung, H. S., Lee, J. -. K. Surface-Plasmon Assisted Energy Conversion in Dye-Sensitized Solar Cells. Advanced Energy Materials. 1 (3), 415-421 (2011).
- Tehrani, S., Chen, E., Durlam, M., DeHerrera, M., Slaughter, J. M., Shi, J., Kerszykowski, G. High density submicron magnetoresistive random access memory (invited). Journal of Applied Physics. 85 (8), 5822-5827 (1999).
- Ross, C. A., et al. Fabrication of patterned media for high density magnetic storage. Journal of Vacuum Science & Technology B. 17, 3168 (1999).
- Gu, M., Zhang, Q., Lamon, S. Nanomaterials for optical data storage. Nature Reviews Materials. 1, 16070 (2016).
- Mock, J. J., Barbic, M., Smith, D. R., Schultz, D. A., Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. The Journal of Chemical Physics. 116 (15), 6755-6759 (2002).
- Su, K. -. H. A., et al. Interparticle Coupling Effects on Plasmon. Resonances of Nanogold Particles, Nano Letters. 3 (8), 1087-1090 (2003).
- Lee, K., El-Sayed, M. A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. The Journal of Physical Chemistry B. 110 (39), 19220-19225 (2006).
- Grzelczak, M., Prez-Juste, J., Mulvaney, P., Liz-Marzn, L. M. Shape control in gold nanoparticle synthesis. Chemical Society Reviews. 37 (9), 1783-1791 (2008).
- Ye, J., Thompson, C. Templated Solid-State Dewetting to Controllably Produce Complex Patterns. Advanced Materials. 23 (13), 1567-1571 (2011).
- Huang, J., Kim, F., Tao, A., Connor, S., Yang, P. Spontaneous formation of nanoparticle stripe patterns through dewetting. Nature Materials. 4, 896-900 (2005).
- Hughes, R. A., Menumerov, E., Neretina, S. When lithography meets self-assembly: a review of recent advances in the directed assembly of complex metal nanostructures on planar and textured surfaces. Nanotechnology. 28 (28), 282002 (2017).
- Kim, D., Giermann, A. L., Thompson, C. V. Solid-state dewetting of patterned thin films. Applied Physics Letters. 95 (25), 251903 (2009).
- Fowlkes, J. D., Doktycz, M. J., Rack, P. D. An optimized nanoparticle separator enabled by electron beam induced deposition. Nanotechnology. 21 (16), 165303 (2010).
- White, B. C. A., et al. The Effect of Different Thickness Alumina Capping Layers on the Final Morphology of Dewet Thin Ni Films. Applied Physics A. 124 (3), 233 (2018).

