Discrimination of Seven Immune Cell Subsets by Two-fluorochrome Flow Cytometry
Summary
Here, we present a flow cytometric protocol to identify CD4+ and CD8+ T cells, γδ T cells, B cells, NK cells and monocytes in human peripheral blood by using only two fluorochromes instead of seven. With this approach, five additional markers can be recorded on most flow cytometers.
Abstract
Immune cell characterization heavily relies on multicolor flow cytometry to identify subpopulations based on differential expression of surface markers. Setup of a classic multicolor panel requires high-end instruments, custom labeled antibodies, and careful study design to minimize spectral overlap. We developed a multiparametric analysis to identify major human immune populations (CD4+ and CD8+ T cells, γδ T cells, B cells, NK cells and monocytes) in peripheral blood by combining seven lineage markers using only two fluorochromes. Our strategy is based on the observation that lineage markers are constantly expressed in a unique combination by each cell population. Combining this information with a careful titration of the antibodies allows investigators to record five additional markers, expanding the optical limit of most flow cytometers. Head-to-head comparison demonstrated that the vast majority of immune cell populations in peripheral blood can be characterized with comparable accuracy between our method and the classic "one fluorochrome-one marker approach", although the latter is still more precise for identifying populations such as NKT cells and γδ T cells. Combining seven markers using two fluorochromes allows for the analysis of complex immune cell populations and clinical samples on affordable 6-10 fluorochrome flow cytometers, and even on 2-3 fluorochrome field instruments in areas with limited resources. High-end instruments can also benefit from this approach by using extra fluorochromes to accomplish deeper flow cytometry analysis. This approach is also very well suited for screening several cell populations in the case of clinical samples with limited number of cells.
Introduction
Flow cytometry is a technique that was developed to analyze multiple parameters on single particles at a rate of several thousand of events per second1. Examples of specimens analyzed by flow cytometry include, but are not limited to, cells, beads, bacteria, vesicles and chromosomes. A fluidic system directs particles at the interrogation point where each particle intersects its path with one or more lasers, and multiple parameters are recorded for further analysis. Forward and side scatters, generated by scattering of the pure laser light, are used to identify the target population and retrieve information about the relative size and internal complexity/granularity of particles, respectively. All the other parameters, that account for most of data in a flow cytometric analysis, are derived by fluorochrome-labeled probes that recognize and bind to specific targets on the particles of interest.
Flow cytometry is a primary tool for immunological studies to identify and characterize cell populations. To dissect the complexity of the immune system, multicolor panels are constantly evolving to expand the number of markers simultaneously recorded for deep immunophenotyping of cell populations1. This is leading to the development of more capable instruments and fluorochromes, with recent high-end flow cytometers exceeding 20 fluorescent parameters. This results in complex study design due to fluorochrome spectral overlap and in higher costs associated with custom antibody labelling and skilled operators. In several instances, complexity and costs are reduced by using separate panels of markers for different cell populations. This approach, however, is error prone, reduces the information in each panel, and can be difficult to apply to samples with limited number of cells. Moreover, increasing the number of markers precludes deep immunophenotyping on instruments with fewer fluorescent parameters. We previously developed a staining protocol to identify major human immune populations (CD4+ and CD8+ T cells, γδ T cells, B cells, NK cells and monocytes) in peripheral blood mononuclear cells (PBMCs) by combining seven lineage markers using only two fluorochromes instead of the seven required using the traditional "one fluorochrome-one marker" approach (www.hcdm.org)2,3. Our initial report explored and validated the notion of combining seven markers in two fluorochromes for deep immunophenotyping. In this report, we present a step-by step protocol to isolate and stain peripheral blood cells, focusing on the practical aspect and troubleshooting steps to achieve a successful staining.
This protocol is based on the observation that lineage markers have a constant expression on the cell surface and that each cell population has an exclusive combination of lineage markers. In PBMCs, CD3 expression subdivides immune cells into two main categories: CD3-positive T lymphocytes and CD3-negative cells. Within the CD3 positive subgroup, CD4+, CD8+ and γδ T cells can be separated using antibodies that solely target CD4, CD8 and the γδ receptor. In a comparable way, within the CD3 negative subgroup, B cells, NK cells and monocytes can be uniquely identified using antibodies against CD19, CD56 and CD14, respectively. In a standard one fluorochrome-one marker approach, anti-CD3, -CD4, -CD8, -CD14, -CD19, -CD56 and -TCR γδ antibodies are detected with seven different fluorochromes. Our approach combines anti-CD3, -CD56, and -TCR γδ antibodies in one fluorochrome (labeled for convenience fluorochrome A) and anti-CD4, -CD8, -CD14 and -CD19 antibodies in a different fluorochrome (fluorochrome B). This is possible by a combination of antibody titration and differential antigen expression. Both CD4+ and CD8+ T cells are positive for the anti-CD3 antibody in fluorochrome A, but they can be separated in fluorochrome B maximizing the expression of the CD8 signal while placing, with an ad hoc titration, the CD4 signal in between the CD8 and the CD3 positive-CD4/CD8 double negative cells. γδ T cells expresses higher level of CD3 than CD4 and CD8, and therefore they can be identified as CD3 high4. This signal is further boosted by labeling γδ T cells in fluorochrome A with an anti-TCR γδ antibodies, thus improving separation between CD3 low T cells and CD3 high γδ T cells. B cells can be identified as CD3– in fluorochrome A and CD19+ in fluorochrome B. To separate CD3 negative NK cells from B cells, an anti-CD56 antibody was used in fluorochrome A as the anti-CD3. This is possible because CD56 expressed on NK cell at a much lower level than CD3 on T cells5. Finally, monocytes can be identified via a combination of forward-side scatter properties and expression of CD14 in fluorochrome B.
The idea of combining up to four markers using two fluorochromes has been already successfully attempted before6,7,8, and has been used in a clinical protocol to identify malignant lymphocytic populations9. A previous report also combined seven markers (with different specificity from the markers than we used in our protocol) using two fluorochromes, but this approach relied on a complex labelling of each antibody with varying amount of fluorochrome10. This is in contrast to our method which uses commercially available antibodies and can be adapted to the instrument configuration and can take advantage of the new generation of polymer fluorochromes.
The overall goal of this methodology is to expand the optical limits of most flow cytometers allowing for the recording of five additional markers to interrogate complex cell populations. As a consequence, advanced immunological analysis can be performed on affordable 6-10 fluorochrome flow cytometers, and 2-3 fluorochrome field instruments can achieve remarkable results in areas with limited resources. High-end instruments can also benefit from this approach by using extra fluorochromes to accomplish deeper flow cytometry analysis and to create modular flow cytometric panels targeting several lineages at the same time11. This can potentially reduce the number of panels used in modular immunophenotyping flow cytometry and reduce costs, errors and handling time. This approach is also very well suited in the case of clinical samples with limited number of cells.
Protocol
All studies of human materials were approved by the Johns Hopkins Institutional Review Board under the Health Insurance Portability and Accountability Act. Patient and control samples were de-identified. PBMCs and blood from healthy controls were obtained by informed consent.
NOTE: This protocol has been tested on freshly or frozen isolated peripheral blood cells and whole blood.
1. Cell Preparation
- Isolation of peripheral blood cells (PBMC) from whole blood
- Draw blood in a 10 mL green-top tube containing sodium heparin. After the tube has been filled with blood, immediately invert the tube several times to prevent coagulation.
NOTE: Tubes containing other anticoagulant such as ethylenediaminetetraacetic acid (EDTA) or sodium citrate can be used with comparable results. If a different amount of blood is collected, the following steps in the protocol should be scaled accordingly. - Carefully transfer drawn blood into a 50 mL conical tube. Dilute the blood with an equal amount of phosphate buffered saline (PBS) without calcium and magnesium.
- Add 15 mL of density gradient medium (e.g., Ficoll) to the bottom of a new 50 mL conical tube and carefully overlay the diluted blood on top of density gradient medium, avoiding any mixing between the density gradient medium and diluted blood.
NOTE: This is a critical step for a proper separation of PBMCs from red cells. - Centrifuge at 400 x g for 30 min at room temperature (RT), with no brake to avoid disruption of the interface.
- After centrifugation, carefully aspirate the upper layer using a pipette and discard it, paying attention to not remove cells at the interface between the plasma and density gradient medium, that is where PBMCs stratifies. Collect as many cells as possible from the interface without touching the red cell pellet at the bottom of the 50 mL conical tube and transfer to a new 50 mL conical tube.
- Add PBS to bring the final volume to 25 mL and invert several times to mix. Centrifuge at 300 x g for 10 min at RT with the brake on to remove any density gradient medium contamination from the cell suspension.
- Carefully aspirate supernatant without disturbing cell pellet. Add PBS to bring the final volume to 25 mL and invert several times to mix. Centrifuge at 200 x g for 10 min at RT with the brake on to remove platelets.
- Carefully aspirate the supernatant without disturbing the cell pellet. Resuspend PBMCs in 1 mL of PBS/0.1% sodium azide. Sodium azide reduces capping, shedding, internalization of the antibodies, and increase cell recovery, but its toxicity can impair cell viability. For this reason, sodium azide should be avoided in all steps if the cells will be cultured for subsequent experiments. Cell isolation and staining without sodium azide gave similar results to staining performed in the presence of sodium azide.
CAUTION: Sodium azide can cause death by affecting the central nervous system. Contact may cause burns to skin and eyes. - Dilute the cells for counting by transferring 30 µL of PBMCs in a 1.5 mL microcentrifuge tube. Then add 120 µL of PBS and 150 µL of Trypan blue to determine cell number and viability (1:10 cell dilution). Resuspend carefully.
- Transfer 10 µL to a hemocytometer, count the cells accordingly to the used counting chamber and determine the number of viable cells. Viable cells = number of Trypan blue negative cells total x 10 (the dilution factor used in this protocol) x 104.
NOTE: On average, 7-15 x 106 of PBMCs should be collected from 10 mL of blood. - Resuspend the cells at 10 x 106 per mL of PBS/0.1% sodium azide
NOTE: PBMC can be frozen and stored in liquid nitrogen for an extended period of time. However, expression of markers such as chemokine receptors can be altered by this procedure.
- Draw blood in a 10 mL green-top tube containing sodium heparin. After the tube has been filled with blood, immediately invert the tube several times to prevent coagulation.
- Preparation of cells from frozen PBMC
NOTE: Different freezing procedures can affect cell recovery and viability12. PBMCs for these experiments were frozen in specially formulated freezing media or fetal bovine serum (FBS)/10% dimethyl sulfoxide (DMSO) with similar results.
CAUTION: DMSO can be slightly hazardous in case of inhalation (lung irritant), skin contact (irritant, permeator), of eye contact (irritant), of ingestion.- Remove frozen PBMC from liquid nitrogen and place on ice. Transfer the cryovial from the ice directly into the 37 °C water bath. Keep the cryovial at water surface and gently shake the vials until a small ice pellet remain. Transfer the cryovial back on ice.
- Remove any water with a 70% ethanol sprayed wipe. Slowly add 1 mL of PBS/0.1% sodium azide at 4 °C and carefully transfer the cell to a 15 mL conical tube. Slowly add cold PBS/0.1% sodium azide at 4 °C to reach a final volume of 15 mL.
- Centrifuge at 300 x g for 10 min. Carefully remove the supernatant, resuspend the cells in 1 mL of PBS/0.1% sodium azide.
NOTE: The cells can also be resuspended in RPMI 10% FBS with similar results. - Dilute the cell for counting by transferring 30 µL of PBMCs in a 1.5 mL microcentrifuge tube. Then add 120 µL of PBS and 150 µL of Trypan blue to determine cell number and viability (1:10 cell dilution). Resuspend carefully.
- Transfer 10 µL to a hemocytometer, count the cells accordingly to the used counting chamber and determine the number of viable cells. Viable cells = number of Trypan blue negative cells total x 10 (the dilution factor used in this protocol) x 104.
- Resuspend the cells at 10 x 106 per mL in PBS/0.1% sodium azide.
- Preparation of cells from whole blood
- Draw blood in a 10 mL green-top tube containing sodium heparin. After the tube has been filled with blood, immediately invert the tube several times to prevent coagulation.
NOTE: Tubes containing other anticoagulant such as EDTA or sodium citrate can be used with comparable results. If a different amount of blood is collected, the following steps in the protocol should be scaled accordingly. - Transfer 200 µL of whole blood to a 12 mm x 75 mm capped tube, add 2 µL of sodium azide and vortex gently for 2 s.
- Draw blood in a 10 mL green-top tube containing sodium heparin. After the tube has been filled with blood, immediately invert the tube several times to prevent coagulation.
2. Cell Staining
NOTE: Choosing pairs of fluorochromes with virtually no spectral overlap is important to reduce spread of data due to high spillover of a fluorochrome in the other fluorochrome detector. To achieve an optimal identification of al the cell subsets, fluorochromes with a high quantum yield should be used such as antibody pairs PE-BV421 and PE-APC.
- Staining of fresh and frozen PBMC
- Transfer 100 µL of PBMC (1 x 106 cells) to a 96-well V-bottom plate.
NOTE: Any number of cells lower than 1 x 106 can be used with similar results2. - Centrifuge at 350 x g for 3 min at RT and carefully aspirate the supernatant without disturbing the cell pellet. Add to each well 100 µL of PBS containing a live/dead fixable dye that reacts with free amine on proteins for 10 min to label dead cells.
- Prepare for each sample 30 µL of a mix containing all the antibodies (anti-CD3. -CD56, TCRγδ in fluorchrome A, and anti-CD4, CD8, CD19, CD14 in fluorchrome B). Concentration of antibodies are indicated in Table 1 and Table2. At this stage, titrated antibodies against different target molecules and in different fluorochromes can be added as well (e.g., Table 3).
NOTE: Antibodies concentration can vary depending on the manufacturer and lot number. Therefore, preliminary tests should be done to achieve the optimal signal. PBS, PBS/0.5% BSA, PBS/0.2% sodium azide or PBS/0.5% BSA/0.1% sodium azide were used with similar results to dilute antibodies2. Cell can also be stained in volumes different from 30 µL to easily integrate this methodology to already existing staining protocols. - Centrifuge at 350 x g for 3 min at RT and carefully aspirate the supernatant without disturbing the cell pellet. Add the antibody cocktail to each well and resuspend carefully without generating bubbles. Incubate for 30 min at RT in the dark.
NOTE: It is possible to stain the samples at 4 °C with similar results. - Add 150 µL of staining buffer and centrifuge at 350 x g for 3 min at RT and carefully aspirate the supernatant without disturbing the cell pellet. Resuspend the cells in 200 µL of PBS and acquire data on a flow cytometer. If staining volumes are changed, please ensure at least a 20-fold dilution of the original antibody mix used to wash the excess of antibodies.
NOTE: Stained cells can be fixed with in PBS/2% paraformaldehyde, kept in a refrigerator at 4 °C overnight and then acquired on a flow cytometer the following day.
CAUTION: Paraformaldehyde is harmful if swallowed and can cause skin irritation
- Transfer 100 µL of PBMC (1 x 106 cells) to a 96-well V-bottom plate.
- Staining of whole blood
- To each 12 mm x 75 mm capped tube, add the cocktail of antibodies (anti-CD3. -CD56, TCRγδ in fluorochrome A, and anti-CD4, CD8, CD19, CD14 in fluorochrome B) and incubate at RT for 30 min at RT in the dark. Concentration of antibodies are indicated in Table 4. At this stage, titrated antibodies against different target molecules and in different fluorochromes can be added as well. Antibody concentration can vary depending on the manufacturer and lot number. Therefore, preliminary tests should be done to achieve the optimal signal.
NOTE: It is possible to stain samples at 4 °C with similar results. - Centrifuge at 350 x g for 3 min at RT and carefully aspirate the supernatant without disturbing the cell pellet. Make a 1x solution of the red blood cell lysis buffer following manufacturer’s instructions.
NOTE: The lysis solution should be at RT before use. - Add 2.0 mL of 1x red blood cell lysis buffer to each tube, cap well and invert several times to mix. Cover with foil and let sit for 15 min.
- Spin down at 300 x g for 5 min. Carefully aspirate the supernatant without disturbing the cell pellet.
- Add 2 mL of PBS and centrifuge at 300 x g for 5 min.
NOTE: At this point, the cell pellet should have a pale white coloration indicating a successful red blood cell lysis. Carefully aspirate the supernatant to not disturb the cell pellet and gently resuspend the cells in 200 µL of PBS. - Strain the cells through 12 mm x 75 mm tubes with 40 µm filter caps to remove cell aggregates and acquire data on a flow cytometer.
- To each 12 mm x 75 mm capped tube, add the cocktail of antibodies (anti-CD3. -CD56, TCRγδ in fluorochrome A, and anti-CD4, CD8, CD19, CD14 in fluorochrome B) and incubate at RT for 30 min at RT in the dark. Concentration of antibodies are indicated in Table 4. At this stage, titrated antibodies against different target molecules and in different fluorochromes can be added as well. Antibody concentration can vary depending on the manufacturer and lot number. Therefore, preliminary tests should be done to achieve the optimal signal.
3. Antibody Titration
NOTE: Antibody titration is the most critical step for obtaining high-quality, reproducible data. Titration of anti-CD3, -CD8, -CD14, -CD19 and -TCR γδ follows the standard procedure by which the concentration of antibody to optimally separate positive and negative peaks is derived by maximum staining index13,14. Dilutions at the peak or closer to the peak on the rising side of the stain index curve should be selected (Figure 1A-C). The anti-CD4 antibody is titrated to place the peak of the CD4 positive population between CD3 single positive populations and CD3+/CD8+ T cells, closer to the CD3 single positive signal to better discriminate the CD8dim populations (CD8+ γδ T cells and NK T cells). Along the same line, CD56 titration aims to position NK CD56+ cells between the CD3+ and the CD3– population.
- Maximum stain index curve
NOTE: Titration of anti-CD3, -CD8, -CD14, -CD19 and -TCR γδ follows the standard procedure by which the concentration of antibody to optimally separate positive and negative peaks is derived by a maximum staining index curve15. If antibodies against other markers are added to the panel, they also need to be titrated with a maximum staining index curve.- Prepare a 2-fold antibody dilution by filling 10 wells of a 96-well plate with 40 µL of staining buffer. In the first well, increase the final volume to 80 µL of staining buffer and add the antibody of interest at a concentration 4 times the concentration suggested by the manufacturer.
- Mix well and transfer 40 µL to the second well. Mix well and repeat this step for the all the other wells.
- Stain 10 samples of PBMC or whole blood with 30 µL of the 10 different 2-fold dilutions of antibodies following the protocol described before.
- Acquire data with a flow cytometer and plot the signal from each dilution (Figure 1A).
- Gate on the negative and positive populations for each antibody concentration. Increasing concentration of antibodies can lead to a higher background. Therefore, resize the negative gate accordingly.
- For each antibody concentration, extract information about the median and standard deviation for the fluorescent intensity of the negative population, and the median for the fluorescent intensity of the positive population. Calculate for each antibody concentration the stain index with this formula: (median fluorescent intensity of the positive population — median fluorescent intensity of the negative population) ÷ (2 x standard deviation of the fluorescent intensity of the negative population) (Figure 1B).
- Plot the stain index vs. the antibody concentration expressed as fraction of the antibody dilution (e.g., 1:10 dilution = 0.1), and identify the concentration of antibody with the maximum stain index value (Figure 1C).
- Anti-CD4 and -CD56 antibody titration
NOTE: Anti-CD4 and -CD56 antibodies titration relies on previous titration the other markers in the two-fluorochrome panel. For the anti-CD4 antibody the titration aims at placing the anti-CD4 signal between the double CD8+/CD3+ signal and the CD3 single positive population (Figure 1D).- Titrate the anti-CD4 and CD56 antibodies with a 2-fold dilution strategy as described before, adding additional concentrations in between to finely identify the range of concentration that allow to separate CD4+ T cells and NK cells from the other cell populations.
- Titrate the anti-CD4 antibody by placing the anti-CD4 signal between the double CD8+/CD3+ signal and the CD3 single positive population (Figure 1D).
NOTE: Special care should be done to clearly separate CD4+ T cells from CD8+ dim populations. - Titrate the anti-CD56 antibody following a strategy similar to the anti-CD4 antibody titration, by placing NK cells between the CD3-negative and the CD3-positive populations.
4. Gating Strategy
- Identify lymphocytic and monocytic cell populations and remove dead cells and most of the residual red blood cells from the analysis.
- Select the entire population containing lymphocytes and monocytes based on forward vs side scatter area (FSC-A vs SSC-A). Remove cell aggregates from the analysis via forward scatter height vs forward scatter width (FSC-H vs FSC-W) and side scatter height vs side scatter width (SSC-H vs SSC-W).
- Use a live/dead discrimination marker to exclude bright positive dead cells and residual red blood cells from the analysis. Gate on lymphocytes and monocytes based on the different FSC-A and SSC-A profile.
- Two-fluorochrome seven-marker gating strategy of the lymphocytic populations.
NOTE: Within the CD3 positive subgroup, CD4+, CD8+ and γδ T cells can be separated using antibodies that solely target CD4, CD8 and the γδ receptor. In a comparable way, within the CD3 negative subgroup, B cells, NK cells and monocytes can be uniquely identified using antibodies against CD19, CD56 and CD14, respectively.- Select the lymphocyte gate and create a dot plot with on each axis one of two fluorochromes used in this protocol (Figure 2B).
- Gate on CD8+ T cells identified as CD3+/CD8+ double positive cells at the top right corner of the dot-plot (Figure 2B). Exclude the dim CD8 population which might contain NKT cells. Gate on CD4+ T cells identified as population in between CD8+ T cells and the CD3 single positive populations. Gate on γδ T cells identified as high CD3 cells. Subdivide γδ T cells in CD8 positive and CD8 negative.
- Gate on NK cells identified as the population in between CD3-positive and CD3-negative cells. Subdivide NK cells in CD8 positive and CD8 negative. Gate on B cells identified as CD3-negative CD19+ population on the right lower corner of the dot plot.
- Select the monocyte gates and create a dot plot with on each axis one of two fluorochromes used in this protocol (Figure 2C). Gate on the CD3–/CD14+ population.
Representative Results
Setup and analysis of a flow cytometry experiment of human peripheral blood cells stained with seven lineage markers (anti-CD3, -CD4, -CD8, -CD14, -CD19, -CD56 and -TCR γδ antibodies) using only two fluorochromes are presented.
Representative results are described for anti-CD8 and -CD56 antibody titration. For each antibody (in this example, anti-CD8), data from ten successive 2-fold dilutions were recorded to calculate a stain index curve (Figure 1A-C). Optimal antibody concentration was determined by the maximum stain index signal13,14. Dilutions at the peak or closer to the peak on the rising side of the stain index curve should be selected. Anti-CD4 and -CD56 antibodies were titrated by staining peripheral blood cells together with the other markers in the two-fluorochrome panel (previously titrated). For the anti-CD4 antibody, the titration should aim at placing the anti-CD4 signal between the double CD8+/CD3+ signal and the CD3 single positive population (Figure 1D). Special care should be done to clearly separate CD4+ T cells from CD8+ dim populations. PBMCs were stained with optimal concentration of the indicated markers and different concentrations of anti-CD4. Color code of the concentrations: green indicates concentrations that result in an optimal separation of CD4+ T cells from the other CD3+populations; orange indicates concentrations that result in an acceptable but not ideal separation; red indicates concentrations that result in poor separation of CD4+ T cells from dim CD8 cells or CD4/CD8 double negative populations. The anti-CD56 titration was done in a similar way as the anti-CD4 antibody, by placing NK cells between the CD3–negative and the CD3-positive populations.
Representative gating strategy shows how to identify lymphocytic and monocytic cell populations and remove from the analysis dead cells and most of the residual red blood cells (Figure 2A). All the subsequent analysis was based on this gating strategy. Representative gating strategy is used to identify CD4+ and CD8+ T cells, γδ T cells, B cells, NK cells and monocytes in the two fluorochrome-seven marker staining (Figure 2B-C).
Representative negative results are deriving from improper sample preparation and titration of anti-CD4 and -CD56 antibodies. Failing to proper titrate CD4 results in poor separation of CD4+ T cells from CD8+ T cells (Figure 3A), while a poor CD56 titration can lead to a poor separation of NK from B cells and cells negative for all the markers in the staining panel (Figure 3B). Poor RBC lysis can occur with whole blood staining. If the primary goal of the protocol is to calculate the percentage of different cell population (e.g., % of CD4+ T cells), contamination with RBC double negative cells, that will appear in the dot plot as double negative population, should be excluded from the analysis (Figure 3C). Based on our experience with this protocol, we have noticed that accurate separation between B cells and NK CD8+ cells can be verified by using other markers. As an example, NK cells are double negative for HLA-DR and CCR6, while B cells are double positive (Figure 3D).
The protocol presented in this manuscript is meant to be part of a multicolor staining panel to interrogate several immune populations in samples with limited number of cells. By using this approach, we investigated dynamics in immune populations of longitudinal samples from donors with multiple myeloma receiving a stem cell transplant (Clinicaltrials.gov NCT0056609816). Frozen PBMC were collected and analyzed by flow cytometry at day 0, 14, 28, 60, 180, 360 after transplantation. By using this approach, we were able to interrogate several lymphocyte populations focusing on their naïve/memory profile (CD45RA, CCR7), activation and cell exhaustion status (HLA-DR, CD57, CD45RA+ effector memory, CD16), and T effector phenotype (CCR4, CCR6, CXCR3) in a single staining panel17,18,19,20,21,22,23,24,25,26. This has been particularly useful considering that the number of collected cells for some of the patients and time points was barely sufficient for only a single staining panel. Representative gating strategy (Figure 4) and dynamics of selected cell populations (Figure 5) of a relapsing patient over time. In multiple myeloma B cells can express the NK marker CD56. To exclude this possibility, we used HLA-DR and CCR6 to further differentiate B cells from NK cells (Figure 4B). CD8+ memory and naïve T cells were identified by the expression of CD45RA and CCR7: naïve (CD45RA+/CCR7+), central memory (CM, CD45RA–/CCR7+), effector memory (EM, CD45RA–/CCR7–) and effector memory CD45RA+ (EMRA, CD45RA+/CCR7+) (Figure 4C). Expression of HLA-DR and CD57 in CD8+ naïve, total memory T cells (which comprise CM, EM and EMRA), CM, EM and EMRA (Figure 4D). CD4+ memory and naïve T cells were identified by the expression of CD45RA and CCR7: naïve (CD45RA+/CCR7+), central memory (CM, CD45RA–/CCR7+), effector memory (EM, CD45RA–/CCR7–) and effector memory CD45RA+ (EMRA, CD45RA+/CCR7+) (Figure 4E). HLA-DR and CD57 expression in CD4+ naïve and memory population (which comprise CM, EM and EMRA), CM, EM and EMRA (Figure 4F). CCR4 and CCR6 were used as markers to identify within the memory population Th9 CD4+ T cells (Figure 4G). Th1, Th1/17, Th2 and Th17 CD4+ T helper subpopulations were identified by expression of CCR4, CCR6 and CXCR3 (Figure 4H). CD16 and CD57 expression in NK cells (Figure 4I). Stem cells transplantation resulted in a sustained CD4+ and CD8+ T cell activation as shown by increased expression of HLA-DR and CD57, and in a skew of T helper to a Th1 phenotype. At day 60 the percentage of B cells dramatically augmented predicting the patient relapse (Figure 5).
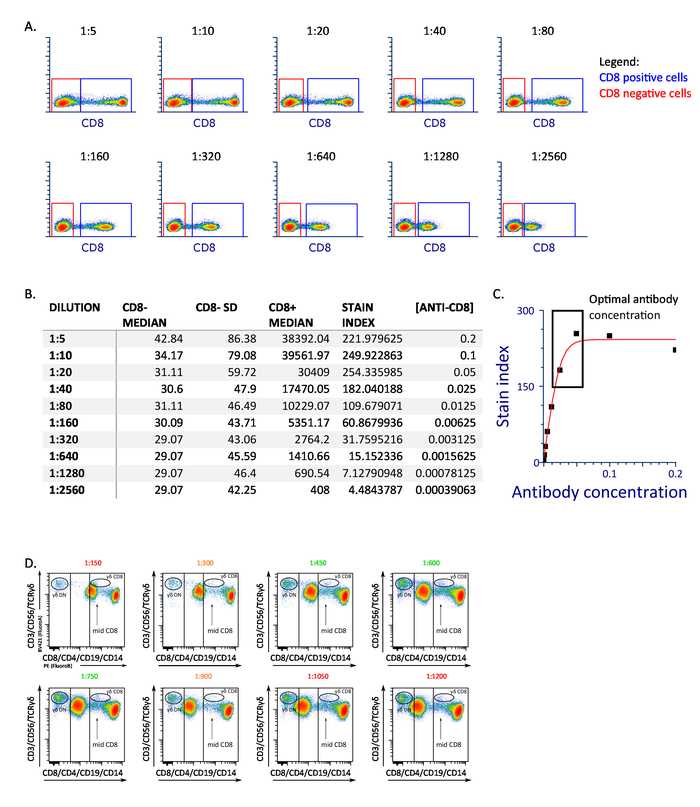
Figure 1: Representative antibody titration. (A) Dot plot shows CD8 expression on fresh PBMC stained with the indicated concentration of the antibody. (B) Table represent the median and standard deviation of fluorescent intensity of the CD8+, median fluorescent intensity CD8– population, and the derived stain index for each concentration tested. (C) The graph shown how to derivate the optimal concentration of the antibody as a function of stain index. (D) Representative titration of CD4 antibody. Panel D has been modified from Boin et al. 20172. Please click here to view a larger version of this figure.
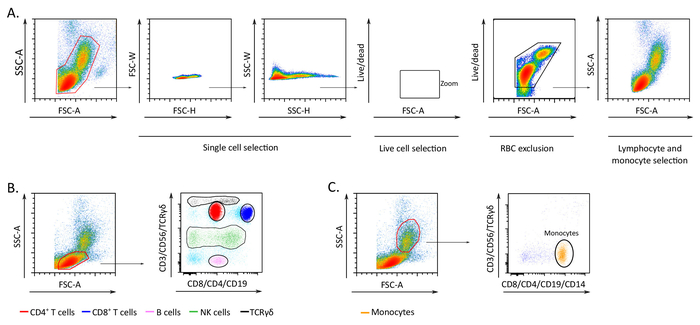
Figure 2: Representative gating strategy and results of subpopulation discrimination. (A) Schematic representation of doublet exclusion, live cells discrimination and size-based gating of lymphocytes and monocytes. (B) Lymphocyte subpopulations identified with the two fluorochrome approach. (C) Monocytes identified with the two fluorochrome approach. The figure has been modified from Boin et al. 20172. Please click here to view a larger version of this figure.
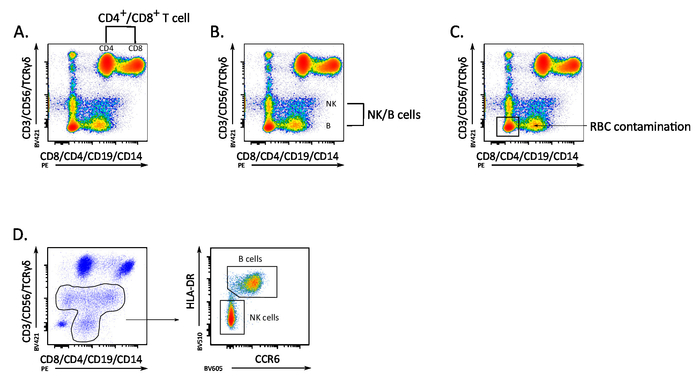
Figure 3: Representative results obtained from improper sample separation and wrong antibody titration. (A) Incorrect titration of CD4 antibody results in poor resolution between CD4+ and CD8+ populations. (B) Poor CD56 titration can lead to a bad separation of NK from B cells. (C) Effect of RBC incomplete lysis on subpopulations discrimination. (D) Example of usage of other markers to verify accurate separation between B cells and NK cells: B cells are HLA-DR and CCR6 double positive, whereas NK cells are double negative. Panel D has been modified from Boin et al. 20172. Please click here to view a larger version of this figure.
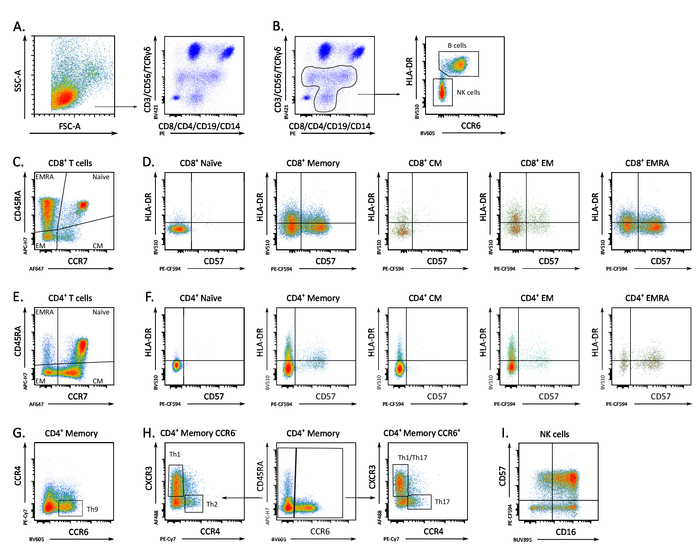
Figure 4: Gating strategy to analyze samples from a patient with multiple myeloma. (A) Lymphocytes were gated on the basis of their FSC-A and SSC-Area and their flow cytometric profile with the two-fluorochrome immune-cell staining is shown. (B) Separation of NK and B cells using CCR6 and HLA-DR. (C) Gating strategy to identify CD8+ memory and naïve T cells. (D) Expression of HLA-DR and CD57 in CD8+ naïve and memory T cells. (E) Gating strategy to identify CD4+ memory and naïve T cells. (F) HLA-DR and CD57 expression in CD4+ naïve and memory T cells. (G) Identification of Th9 CD4+ T cells (H) Identification of Thelper CD4+ T cell subpopulations (I) CD16 and CD57 expression in NK cells. The figure has been adapted from Boin et al. 20172. Please click here to view a larger version of this figure.
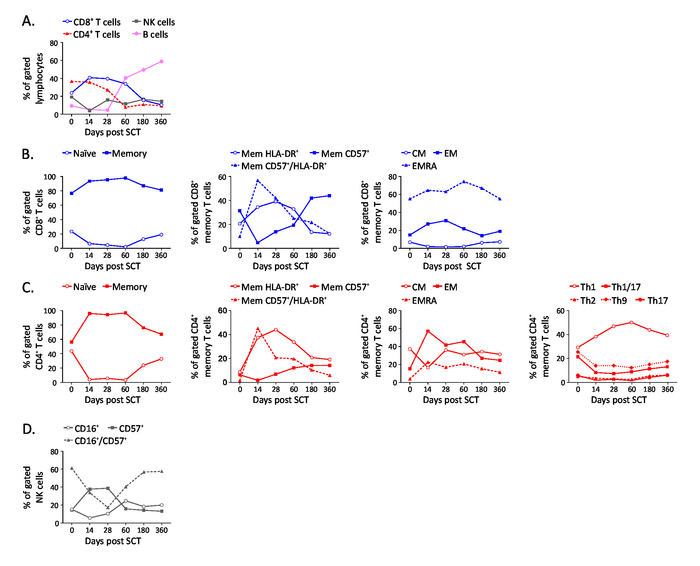
Figure 5: Dynamics of cell population in patient with multiple myeloma. (A) Dynamic of major lymphocyte populations in PBMC isolated and cryopreserved at the indicated day after stem cell transplant (SCT). (B) Characterization of CD8 subpopulations over time. (C) Characterization of CD4 subpopulations over time. (D) Analysis of NK subsets. Data have been plotted with GraphPad Prism. The figure has been adapted from Boin et al. 20172. Please click here to view a larger version of this figure.
| Target | Clone | Fluorochrome | Vendor | Concentration | Purpose |
| CD3 | UCHT1 | BV421 | BD | 1/20 | Lineage |
| CD56 | NCAM16.2 | BV421 | BD | 1/900 | |
| TCRγδ | B1 | BV421 | Bio | 1/30 | |
| CD4 | RPA-T4 | PE | BD | 1/450 | |
| CD8 | RPA-T8 | PE | BD | 1/20 | |
| CD14 | M5E2 | PE | BD | 1/15 | |
| CD19 | HIB19 | PE | BD | 1/300 | |
| Dead cells | L/D Blue | LT | 1/300 | Live/Dead discrimination | |
| BD = BD Biosciences, Bio = BioLegend, LT = Life Technologies | |||||
Table 1: Antibody panel used for the two-fluorochrome immune-cell staining of PBMC (BV421-PE combination).
| Target | Clone | Fluorochrome | Vendor | Concentration | Purpose |
| CD3 | UCHT1 | APC | BD | 1/20 | Lineage |
| CD56 | NCAM16.2 | APC | BD | 1/60 | |
| TCRγδ | B1 | APC | Bio | 1/30 | |
| CD4 | RPA-T4 | PE | BD | 1/450 | |
| CD8 | RPA-T8 | PE | BD | 1/20 | |
| CD14 | M5E2 | PE | BD | 1/15 | |
| CD19 | HIB19 | PE | BD | 1/300 | |
| Dead cells | L/D Blue | LT | 1/300 | Live/Dead discrimination | |
| BD = BD Biosciences, Bio = BioLegend, LT = Life Technologies | |||||
Table 2: Antibody panel used for the two-fluorochrome immune-cell staining of PBMC (APC-PE combination).
| Target | Clone | Fluorochrome | Catalog | Vendor | Concentration | Purpose |
| CD3 | UCHT1 | BV421 | 562426 | BD | 1/20 | Lineage |
| CD56 | NCAM16.2 | BV421 | 562751 | BD | 1/900 | |
| TCRγδ | B1 | BV421 | 331217 | Bio | 1/30 | |
| CD4 | RPA-T4 | PE | 555347 | BD | 1/450 | |
| CD8 | RPA-T8 | PE | 555367 | BD | 1/20 | |
| CD14 | M5E2 | PE | 555398 | BD | 1/15 | |
| CD19 | HIB19 | PE | 555413 | BD | 1/300 | |
| CCR7 | G043H7 | AF647 | 353217 | Bio | 1/30 | Differentiation |
| CD45RA | HI100 | APC-H7 | 560674 | BD | 1/60 | |
| CCR4 | 1G1 | PE-Cy7 | 561034 | BD | 1/60 | Th subsets |
| CCR6 | G034-E3 | BV605 | 353419 | Bio | 1/30 | |
| CXCR3 | 1C6/CXCR3 | AF488 | 561730 | BD | 1/30 | |
| CD57 | NK-1 | PE-CF594 | 562488 | BD | 1/900 | Activation/Exhaustion |
| HLA-DR | G46-6 | BV510 | 563083 | BD | 1/30 | |
| CD16 | 3G8 | BUV395 | 563784 | BD | 1/30 | NK, Monocyte activation |
| Dead cells | L/D Blue | L-23105 | LT | 1/300 | Live/Dead discrimination | |
| BD = BD Biosciences, Bio = BioLegend, LT = Life Technologies | ||||||
Table 3: Antibody panel used to stain frozen PBMC from a patient with multiple myeloma.
| Target | Clone | Fluorochrome | Vendor | Concentration | Purpose |
| CD3 | UCHT1 | BV421 | BD | 1/80 | Lineage |
| CD56 | NCAM16.2 | BV421 | BD | 1/400 | |
| TCRγδ | B1 | BV421 | Bio | 1/200 | |
| CD4 | RPA-T4 | PE | BD | 1/1200 | |
| CD8 | RPA-T8 | PE | BD | 1/100 | |
| CD14 | M5E2 | PE | BD | 1/80 | |
| CD19 | HIB19 | PE | BD | 1/300 | |
| Dead cells | L/D Blue | LT | 1/300 | Live/Dead discrimination | |
| BD = BD Biosciences, Bio = BioLegend, LT = Life Technologies | |||||
Table 4: Antibody panel used for the two-fluorochrome immune-cell staining of whole blood (BV421-PE combination).
Discussion
The protocol presented here has been shown to be quite flexible and insensitive to changes in staining buffer, temperature and peripheral blood cell preparation due to the high expression of lineage markers on the cell surface. The most critical step for obtaining high-quality, reproducible data is antibody titration. Of note, since the titration of antibodies should always be performed during the setup of a flow cytometric panel, this step does not add extra bench-time to our two-fluorochrome approach. Titration of anti-CD3, -CD8, -CD14, -CD19 and -TCR γδ follows the standard procedure by which the concentration of antibody to optimally separate positive and negative peaks is derived by maximum staining index13,14. Dilutions at the peak or closer to the peak on the rising side of the stain index curve should be selected (Figure 1A-C). On the other hand, an ad hoc titration of anti-CD4 and anti-CD56 antibodies needs to be performed. The anti-CD4 antibody is titrated to place the peak of the CD4 positive population between CD3 single positive populations and CD3+/CD8+ T cells, closer to the CD3 single positive signal to better discriminate the CD8dim populations (CD8+ γδ T cells and NK T cells). Along the same line, CD56 titration aims to position NK CD56+ cells between the CD3+ and the CD3 population. The naturally lower expression of CD56 makes the titration of this antibodies easier with the concentration to use close to the value obtained in a saturation curve. Using high quantum yield fluorochromes is another critical factor for an optimal separation of multiple markers/populations on the same detector. We obtained successful results with APC, BV421 and PE, but other fluorochromes, such as the new generation of polymer dye, should give comparable results. To decrease the possibility of artifacts due to compensation, it is also important to choose a pair of fluorochromes with little, if any, spectral overlap, such as PE and APC, or PE and BV421. Choosing pairs of fluorochromes with virtually no compensation is important to reduce spread of data due to high spillover of a fluorochrome in the other fluorochrome detector. Spreading reduction facilitates gating immune subpopulations by minimizing signal distortion and allows to use this methodology, if limited to two fluorochromes, without need of compensation controls.
The combination of markers that we proposed is highly customizable based on the investigator requirements. Indeed, some of the markers can be excluded from the analysis if they do not refer to a population of interest. For example, it is possible to remove the anti-CD19 antibody to exclude B cells, or the anti-CD4 antibody to focus only on the CD8+ T cells. Of note, anti-CD8 antibody is important to identify CD8+ NK cells and γδ T cells, and therefore should not be removed from the panel. To improve the separation of rare cell populations, other fluorochromes/detectors can be used for some of the markers of the two-fluorochrome staining. As an example, CD56 can be moved to a different detector to detect NKT cells, which is not possible with the two-fluorochrome panel. While it is possible to reduce the number of markers from the panel, caution should be exerted in adding, changing or switching markers.
Provided the necessary instrumentation, antibodies and skill set, the standard one-fluorochrome-one marker approach is still the most accurate way to identify multiple immune populations and discriminate rare subpopulations, such as NKT or γδ T cells. However, the primary goal of this method is not to substitute for the classic approach, but rather to achieve a deep immunophenotyping when working with instruments with a low number of detectors, or samples with limited numbers of cells, while reducing complexity and cost in setting up the experimental system. We have done extensive screening of clinical samples from patients with multiple myeloma, systemic sclerosis, dermatomyositis and Lyme disease showing that this staining procedure can improve simultaneous interrogation of several populations with limited number of cells. Our results so far have shown that this procedure is insensitive to chronic immune activation or infectious disease, but preliminary testing should be conducted to assess the accuracy of this protocol in different disease states.
Future directions to further strengthen the potential of this protocol include studies to characterize infiltrating lymphocytes in primary tissues from clinical samples. This is relevant for tumor and autoimmune disease immunology where this approach could provide invaluable information for the analysis of specimens with limited material. We are planning to test this panel on permeabilized cells to expand the potentiality to detect cytokine expression and signaling molecules on the same clinical samples. Finally, it should be noted that similar approaches, aimed at expanding the number of recordable markers, could also be developed using different sets of markers and can also be developed fordifferent animal models.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, <https://www.niams.nih.gov/>, award number P30-AR053503; The Stabler Foundation, www.stablerfoundation.org; National Institute of Allergy and Infectious Disease, www.niaid.nih.gov, T32AI007247; Nina Ireland Program for Lung Health (NIPLH), https://pulmonary.ucsf.edu/ireland/.
Materials
| CD3 | BD Biosciences | 562426 | Antibody for staining RRID: AB_11152082 |
| CD56 | BD Biosciences | 562751 | Antibody for staining RRID: AB_2732054 |
| TCRgd | BD Biosciences | 331217 | Antibody for staining RRID: AB_2562316 |
| CD4 | BD Biosciences | 555347 | Antibody for staining RRID: AB_395752 |
| CD8 | BD Biosciences | 555367 | Antibody for staining RRID: AB_395770 |
| CD14 | BD Biosciences | 555398 | Antibody for staining RRID: AB_395799 |
| CD19 | BD Biosciences | 555413 | Antibody for staining RRID: AB_395813 |
| CD3 | BD Biosciences | 555335 | Antibody for staining RRID: AB_398591 |
| CD56 | BD Biosciences | 555518 | Antibody for staining RRID: AB_398601 |
| TCRgd | BD Biosciences | 331211 | Antibody for staining RRID: AB_1089215 |
| CCR7 | Biolegend | 353217 | Antibody for staining RRID: AB_10913812 |
| CD45RA | BD Biosciences | 560674 | Antibody for staining RRID: AB_1727497 |
| CCR4 | BD Biosciences | 561034 | Antibody for staining RRID: AB_10563066 |
| CCR6 | BD Biosciences | 353419 | Antibody for staining RRID: AB_11124539 |
| CXCR3 | BD Biosciences | 561730 | Antibody for staining RRID: AB_10894207 |
| CD57 | BD Biosciences | 562488 | Antibody for staining RRID: AB_2737625 |
| HLA-DR | BD Biosciences | 563083 | Antibody for staining RRID: AB_2737994 |
| CD16 | BD Biosciences | 563784 | Antibody for staining RRID: AB_2744293 |
| Dead cells | Life technologies | L-23105 | Live/dead discrimination |
| Falcon 5 ml round-bottom polystyrene test tube with cell strainer snap cap | BD Bioscience | 352235 | to filter cell suspension before passing though the flow cytometer |
| Falcon 5 ml round-bottom polystyrene test tube | BD Bioscience | 352001 | to stain whole blood |
| Recovery Cell Culture Freezing Medium | Thermo fisher | 12648010 | Freezing cells |
| 96-well V-bottom plate | Thermo fisher | 249570 | plate for staining |
| FACSAria IIu Cell Sorter | BD Biosciences | Flow cytometer | |
| FCS Express 6 | De Novo Software | FACS analysis | |
| Graphpad Prism | GraphPad software | Data analysis |
References
- Bendall, S. C., Nolan, G. P., Roederer, M., Chattopadhyay, P. K. A deep profiler’s guide to cytometry. Trends in Immunology. 33 (7), 323-332 (2012).
- Boin, F., et al. Flow cytometric discrimination of seven lineage markers by using two fluorochromes. PLoS ONE. 12 (11), (2017).
- Zola, H., et al. . Leukocyte and Stromal Cell Molecules: The CD Markers. , (2007).
- Lambert, C., Genin, C. CD3 bright lymphocyte population reveal γδ T cells. Cytometry Part B: Clinical Cytometry. 61 (1), 45-53 (2004).
- Ginaldi, L., et al. Differential expression of T cell antigens in normal peripheral blood lymphocytes: a quantitative analysis by flow cytometry. Journal of Clinical Pathology. 49 (7), 539-544 (1996).
- Park, J., Han, K. Single-color Multitarget Flow Cytometry Using Monoclonal Antibodies Labeled with Different Intensities of the Same Fluorochrome. Annals of Laboratory Medicine. 32 (3), 171-176 (2012).
- Mansour, I., et al. Triple labeling with two-color immunoflorescence using one light source: A useful approach for the analysis of cells positive for one label and negative for the other two. Cytometry. 11 (5), 636-641 (1990).
- Bocsi, J., Melzer, S., Dähnert, I., Tárnok, A. OMIP-023: 10-Color, 13 antibody panel for in-depth phenotyping of human peripheral blood leukocytes. Cytometry Part A. 85 (9), 781-784 (2014).
- Van Dongen, J. J. M., et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 26 (9), 1908-1975 (2012).
- Bradford, J. A., Buller, G., Suter, M., Ignatius, M., Beechem, J. M. Fluorescence-intensity multiplexing: Simultaneous seven-marker, two-color immunophenotyping using flow cytometry. Cytometry Part A. 61 (2), 142-152 (2004).
- Rühle, P. F., Fietkau, R., Gaipl, U. S., Frey, B. Development of a Modular Assay for Detailed Immunophenotyping of Peripheral Human Whole Blood Samples by Multicolor Flow Cytometry. International Journal of Molecular Sciences. 17 (8), (2016).
- Hønge, B. L., Petersen, M. S., Olesen, R., Møller, B. K., Erikstrup, C. Optimizing recovery of frozen human peripheral blood mononuclear cells for flow cytometry. PLOS ONE. 12 (11), e0187440 (2017).
- Roederer, M. K. FACS analysis of lymphocytes. Handbook of Experimental Immunology. 49, (1997).
- Srivastava, P., Sladek, T. L., Goodman, M. N., Jacobberger, J. W. Streptavidin-based quantitative staining of intracellular antigens for flow cytometric analysis. Cytometry. 13 (7), 711-721 (1992).
- Maecker, H. T., et al. Selecting fluorochrome conjugates for maximum sensitivity. Cytometry Part A. , (2004).
- Noonan, K. A., et al. Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Science Translational Medicine. 7 (288), (2015).
- Mahnke, Y. D., Beddall, M. H., Roederer, M. OMIP-017: Human CD4+ helper T-cell subsets including follicular helper cells. Cytometry Part A. 83 (5), 439-440 (2013).
- Bonecchi, R., et al. Differential Expression of Chemokine Receptors and Chemotactic Responsiveness of Type 1 T Helper Cells (Th1s) and Th2s. The Journal of Experimental Medicine. 187 (1), 129-134 (1998).
- Acosta-Rodriguez, E. V., et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature Immunology. 8 (6), 639-646 (2007).
- Rivino, L., et al. Chemokine Receptor Expression Identifies Pre-T Helper (Th)1, Pre-Th2, and Nonpolarized Cells among Human CD4+ Central Memory T Cells. The Journal of Experimental Medicine. 200 (6), 725-735 (2004).
- Ye, Z. -. J., et al. Differentiation and recruitment of Th9 cells stimulated by pleural mesothelial cells in human Mycobacterium tuberculosis infection. PloS One. 7 (2), e31710 (2012).
- Speiser, D. E., et al. Human CD8+ T cells expressing HLA-DR and CD28 show telomerase activity and are distinct from cytolytic effector T cells. European Journal of Immunology. 31 (2), 459-466 (2001).
- Caruso, A., et al. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry. 27 (1), 71-76 (1997).
- Brenchley, J. M., et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 101 (7), 2711-2720 (2003).
- Palmer, B. E., Blyveis, N., Fontenot, A. P., Wilson, C. C. Functional and Phenotypic Characterization of CD57+CD4+ T Cells and Their Association with HIV-1-Induced T Cell Dysfunction. The Journal of Immunology. 175 (12), 8415-8423 (2005).
- Focosi, D., Bestagno, M., Burrone, O., Petrini, M. CD57+ T lymphocytes and functional immune deficiency. Journal of Leukocyte Biology. 87 (1), 107-116 (2010).

